Cisplatin induced Emesis Modeling & Pharmacodynamics Service
Creative Biolabs offers a range of well-established models to assess the efficacy of anti-emesis treatments. These models, including both in vivo and in vitro systems, are customized to meet specific research needs, ensuring a comprehensive evaluation of drug candidates aimed at managing or preventing emesis.
Introduction
Emesis, commonly known as vomiting, is a complex and protective reflex that involves the forceful expulsion of stomach contents through the mouth. It can be triggered by various factors, including infections, toxins, medications (e.g., chemotherapy), motion sickness, or underlying diseases. The central nervous system, particularly the brainstem’s vomiting center, plays a crucial role in coordinating this response. The physiological mechanisms of emesis involve sensory inputs from the gastrointestinal, vestibular, and other systems, which are processed by the brain to initiate the vomiting reflex. Emesis can manifest as acute or chronic vomiting, depending on the duration and underlying cause. Acute emesis often results from infections, toxins, or sudden gastrointestinal disturbances, whereas chronic vomiting may be linked to more persistent conditions such as gastrointestinal disorders, neurological issues, or as a side effect of long-term medication use. Additionally, some individuals may experience cyclic vomiting syndrome, where episodes of intense vomiting occur without an identifiable cause.
Disease Models and Applications
The Cisplatin-Induced Emesis Model is a widely used preclinical model to evaluate anti-emetic drugs, particularly in the context of chemotherapy-induced nausea and vomiting (CINV). This model typically involves administering a single dose of cisplatin, a chemotherapy agent known for its potent emetogenic effects, to rodents (usually mice or rats). Following administration, animals typically exhibit a significant increase in vomiting and other symptoms such as abdominal contractions and pica. The model mimics the human condition of CINV, making it a valuable tool for screening potential antiemetic treatments. One of the primary advantages of this model is its reproducibility and ability to simulate both acute and delayed phases of emesis observed in clinical settings. Additionally, the model provides insight into the physiological mechanisms behind emesis, such as central nervous system activation and the role of neurotransmitters like serotonin. However, the model also has limitations, including its reliance on animal models that may not fully replicate human emetic responses. Furthermore, cisplatin-induced emesis is not a perfect model for all types of emesis, such as those induced by motion sickness or other non-chemotherapy causes. Despite these limitations, the model remains essential in evaluating novel antiemetic therapies.
Simulates: The cisplatin-induced emesis model simulates chemotherapy-induced nausea and vomiting (CINV), a common and debilitating side effect experienced by cancer patients undergoing treatment with cisplatin and other chemotherapy agents. This model replicates both the acute and delayed phases of emesis observed in human patients, making it a valuable tool for studying the mechanisms of emesis in the context of chemotherapy.
Evaluates Drugs: This model is primarily used to evaluate the efficacy of antiemetic drugs aimed at preventing or reducing chemotherapy-induced nausea and vomiting. It is used to screen potential therapies targeting various pathways involved in emesis, such as serotonin receptor antagonists (e.g., ondansetron), NK1 receptor antagonists (e.g., aprepitant), and other novel agents designed to alleviate the discomfort and distress caused by CINV.
Measurements
We offer a variety of measurements for evaluating drug efficacy in the cisplatin-induced emesis model, utilizing an array of advanced technologies, including but not limited to:
- General observations: frequency and severity of vomiting, abdominal contractions, and pica behavior.
- Video analysis: Monitoring and recording emetic episodes to assess the timing and duration of vomiting, providing detailed behavioral data.
- Immunohistochemistry: Detection of activation of specific receptors (e.g., serotonin receptors, NK1 receptors) in brainstem regions involved in emesis.
- Cytokine profiling (e.g., ELISA): Measurement of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in serum or gastrointestinal tissues, to evaluate inflammation levels.
- Hematology analysis and serum biomarkers: Assessing liver function and general health status through parameters such as serum electrolytes, liver enzymes, and creatinine levels.
- Gene/protein expression profiling via RT-qPCR and Western blot: Quantification of gene and protein expression related to emetic pathways (e.g., serotonin transporter, substance P, or NK1 receptor).
In addition to the established cisplatin-induced emesis model, our expertise extends to customizing models tailored to specific research needs, guided by literature and prior studies. Our scientific team is available to assist in experimental design, model selection, and data analysis, ensuring a personalized and effective approach to your project at every stage.
Related Services
In addition to the cisplatin-induced emesis model, we also offer various other emesis models induced by different methods to accommodate a wide range of research needs.
Advantages
- Expertise in Multiple Therapeutic Areas: We specialize in a broad range of diseases, including cancer, fibrosis, cardiovascular diseases, and neurological conditions, offering expertise across diverse drug development areas.
- Cutting-Edge Technology: We utilize the latest advancements in technology, from advanced imaging techniques to high-throughput screening methods, ensuring precise and accurate results for all studies.
- Customizable Models and Services: Our models are flexible and can be tailored to meet the unique requirements of each research project, whether you're working on drug efficacy, safety, or mechanism of action studies.
- Comprehensive Support: We provide full-service support throughout the research process, from experimental design to data analysis, helping to optimize research outcomes and accelerate timelines.
- Regulatory Compliance: Our work adheres to the highest standards of quality control and regulatory compliance, ensuring reliability and consistency in every study.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
Q1: What is the cisplatin-induced emesis model used for?
A1: The cisplatin-induced emesis model is primarily used to study chemotherapy-induced nausea and vomiting (CINV) and evaluate the efficacy of antiemetic drugs. It simulates the acute and delayed phases of emesis observed in cancer patients undergoing chemotherapy treatment.
-
Q2: What are the main advantages of the cisplatin-induced emesis model?
A2: This model offers high reproducibility, mimicking human emesis patterns caused by chemotherapy. It helps in evaluating antiemetic drugs and understanding the mechanisms behind nausea and vomiting induced by chemotherapeutic agents.
-
Q3: Can the cisplatin-induced emesis model be used for all types of emesis research?
A3: No, this model specifically mimics chemotherapy-induced emesis. For other types of emesis, such as motion sickness or radiation-induced nausea, we offer alternative models tailored to those conditions.
-
Q4: How do you monitor emesis in this model?
A4: Emesis is monitored through general observations such as vomiting frequency, abdominal contractions, and behavior analysis. Additionally, video monitoring and histological analysis are employed for more detailed insights into the underlying mechanisms.
-
Q5: What types of measurements are used to assess drug efficacy in this model?
A5: We use a variety of methods, including general behavioral observations, immunohistochemistry, cytokine profiling, hematology analysis, and gene/protein expression profiling, to evaluate the efficacy of anti-emetic drugs.
-
Q6: Can this model be customized for specific research needs?
A6: Yes, we offer customized Cisplatin-Induced Emesis Models, with tailored experimental designs, drug treatments, and endpoint evaluations to match your specific research objectives.
Published Data
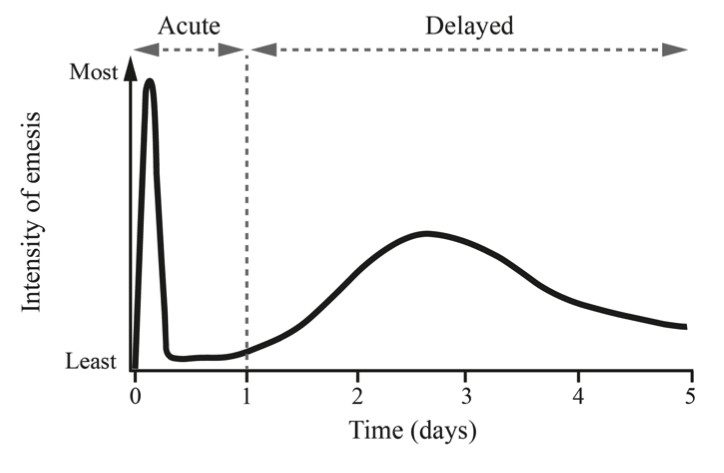 Fig.1 Pattern of cisplatin-induced delayed emesis.1
Fig.1 Pattern of cisplatin-induced delayed emesis.1
This experiment aims to illustrate the typical biphasic pattern of chemotherapy-induced nausea and vomiting (CINV) observed following high-dose cisplatin administration. As shown in Figure 1, the first peak of emesis occurs within the first 24 hours, characterized by intense nausea and vomiting. A secondary, less intense peak is observed on days 2 and 3, where symptoms of nausea and vomiting persist at a reduced intensity. This pattern is critical for understanding the time-dependent progression of CINV and is essential for evaluating the effectiveness of antiemetic treatments in preclinical studies.
Reference
- Rapoport, Bernardo L. "Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management." Frontiers in Pharmacology vol. 8 19. 30 Jan. 2017, DOI:10.3389/fphar.2017.00019. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
