Introduction: Deciphering Cellular Communication at Single Cell Resolution
The immune system operates through a complex and dynamic network of cellular communication, orchestrated largely by cytokines. These soluble mediators dictate the nature, magnitude, and duration of an immune response. Historically, quantifying these proteins via bulk methods like ELISA provided a valuable, yet incomplete, snapshot of systemic levels. Such approaches measure the average secretion from a heterogeneous population, masking the contributions of rare but potent cellular subsets and failing to identify the specific phenotype of the cytokine-producing cells.
To achieve a profound understanding of cellular function—essential for developing next-generation vaccines, immunotherapies, and cell-based medicines—a more granular approach is required. Intracellular Cytokine Staining (ICS) coupled with multicolor flow cytometry has emerged as the definitive technology for this purpose. This powerful technique dissects complex immune responses at the single cell level, simultaneously defining a cell's lineage, differentiation state, and functional capacity. It allows researchers to ask not just what cytokines are produced, but precisely which cells are producing them, and in what combinations, providing unparalleled insight into the mechanisms of health and disease.
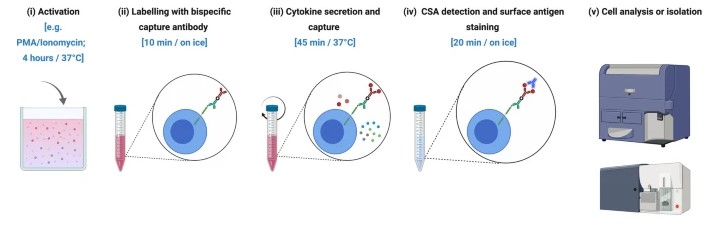 Fig.1 Flow cytometry workflow for the cytokine secretion assay.1
Fig.1 Flow cytometry workflow for the cytokine secretion assay.1
In Vitro Functional and Intracellular Cytokine Analysis by Multicolor Flow Cytometry at Creative Biolabs
At Creative Biolabs, the ICS assay is a cornerstone of our functional immunology services, refined over two decades to deliver data of the highest caliber. Our procedure transforms a static cellular phenotype into a dynamic functional readout through a meticulously controlled workflow, where precision at each stage is paramount.
Ex Vivo and In Vitro Cellular Activation
The analytical journey begins with the controlled stimulation of live cells to induce cytokine synthesis. The choice of stimulant is critical and tailored to the specific biological question:
-
Antigen-Non-specific Stimulation: For assessing the maximal cytokine-producing potential of a cell population, we employ potent mitogens like Phorbol 12-myristate 13-acetate (PMA) and Ionomycin. This provides a robust, global assessment of cellular functional capacity.
-
Antigen-Specific Stimulation: To probe for rare, antigen-responsive cells, especially in vaccine and immuno-oncology studies, we culture cells with specific peptide pools, recombinant proteins, or whole-pathogen lysates. This approach allows us to precisely identify and characterize the functional signature of the specific cells responding to the therapeutic or pathogen.
Cytokine Secretion Blockade: The Critical Accumulation Step
Following activation, newly synthesized cytokines are rapidly trafficked for secretion. To enable intracellular detection, this pathway must be arrested. We utilize optimized protein transport inhibitors, which force cytokines to accumulate within the cell to a concentration that is readily detectable. The selection and timing of this blockade are crucial variables that Creative Biolabs has perfected to maximize signal-to-noise ratios without inducing significant cytotoxicity.
Immunostaining: Crafting a Multi-Layered Phenotypic Portrait
With cytokines trapped internally, we execute a sequential, multi-layered staining protocol:
-
Viability Staining: We first apply a viability dye to exclude dead cells, an essential step to prevent the confounding artifacts that arise from non-specific antibody binding to compromised cells.
-
Surface Marker Staining: A meticulously titrated cocktail of fluorochrome-conjugated antibodies targeting surface antigens (e.g., CD3, CD4, CD8, CD19, CD56) is applied to define each cell's lineage and differentiation state.
-
Fixation and Permeabilization: Cells are fixed to preserve their structural integrity and then gently permeabilized using validated reagents that create pores in the membrane without denaturing critical intracellular epitopes.
-
Intracellular Staining: A final antibody cocktail targeting the intracellular cytokines (e.g., IFN-γ, TNF-α, IL-2, IL-17A) and key transcription factors (e.g., FoxP3 for regulatory T cells) is introduced, completing the multi-parameter cellular profile.
Data Acquisition and Analysis
The stained cells are analyzed on our advanced flow cytometers. The resulting high-dimensional data is then subjected to a rigorous analysis pipeline. This involves a systematic gating strategy to identify canonical cell populations, which are then interrogated for cytokine expression.
Our Service Content
Creative Biolabs provides a comprehensive suite of ICS services engineered to meet the rigorous demands of preclinical research and regulated clinical trial programs.
-
Comprehensive Immunophenotyping: Baseline characterization of all major immune cell lineages from whole blood, PBMCs, or dissociated tissue.
-
T Cell, B Cell, and NK Cell Functional Analysis: Targeted assays to measure key cytokine production (e.g., IFN-γ, TNF-α, IL-2, IL-4, IL-10, IL-17A) in lymphocyte populations.
-
Polyfunctionality Assessment: A critical metric for therapeutic efficacy, our assays precisely quantify the percentage of cells co-expressing multiple effector functions, identifying the most potent cellular responders.
-
T Cell Differentiation and Activation Profiling: Simultaneous analysis of differentiation markers (e.g., CD45RA, CCR7) and activation markers (e.g., CD69, CD25) alongside cytokine production.
-
Regulatory T Cell (Treg) Analysis: Staining for the master transcription factor FoxP3 in combination with cytokine analysis to assess the crucial balance between effector and regulatory responses.
-
Custom Panel Development: Our scientific team collaborates directly with clients to design, optimize, and validate bespoke antibody panels for unique biological questions, accommodating up to 15 or more parameters.
Our Advantages
With over two decades of specialized expertise, Creative Biolabs delivers more than just data; we provide actionable insights backed by unparalleled quality and scientific rigor.
-
Deep Scientific Expertise
-
State-of-the-Art Technology
-
Rigorous Standardization
-
Customization and Flexibility
FAQs
-
What is the fundamental difference between ICS and a bulk assay like ELISA?
ELISA measures the total concentration of a secreted cytokine in a sample supernatant, providing an average readout from all cells. ICS is a single cell technique. It identifies precisely which cells are producing the cytokine(s) of interest and can quantify how many different cytokines a single cell produces (polyfunctionality), providing a much deeper, more mechanistic understanding of the immune response.
-
Can you perform ICS analysis on cryopreserved samples?
Yes. While fresh samples are often considered the gold standard, we have fully validated protocols for using cryopreserved PBMCs. We understand the logistical necessity of cryopreservation in multi-site clinical trials and have optimized our procedures to minimize viability loss and functional alterations, ensuring the highest possible data quality from banked samples.
-
How many cells are typically required for an ICS assay?
The required cell number depends on the frequency of the target population. For analyzing common T cell responses, 1-2 million PBMCs per stimulation condition is standard. For detecting very rare antigen-specific cells, higher cell numbers may be required. Our team will provide a precise recommendation during the project consultation.
-
What is "polyfunctionality," and why is it important?
Polyfunctionality refers to the ability of a single cell, typically a T cell, to co-express multiple effector cytokines (e.g., IFN-γ, TNF-α, and IL-2) simultaneously. A high degree of polyfunctionality is widely considered a hallmark of a highly effective and durable immune response and is a critical endpoint in the evaluation of vaccines and immunotherapies.
Advance your research with the leaders in functional cellular analysis. To discuss how our intracellular cytokine staining services can empower your next project, contact the scientific team at Creative Biolabs today.
Reference
-
Rezk, Ayman et al. "Multiplexed detection and isolation of viable low-frequency cytokine-secreting human B cells using cytokine secretion assay and flow cytometry (CSA-Flow)." Scientific Reports vol. 10,1 14823. 9 Sep. 2020, doi:10.1038/s41598-020-71750-z. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.

 Fig.1 Flow cytometry workflow for the cytokine secretion assay.1
Fig.1 Flow cytometry workflow for the cytokine secretion assay.1