We begin by engineering your chosen target cell lines to stably co-express the specific target antigen and a highly sensitive reporter gene (e.g., luciferase). This ensures a direct and quantifiable readout of cell viability upon target cell lysis.
TDCC Reporter Assay Service
Advancing Gene and Cell Therapy with TDCC Reporter Assay
The landscape of gene and cell therapy is rapidly evolving, bringing forth groundbreaking therapeutic modalities like CAR-T cells, bispecific antibodies, and antibody-drug conjugates (ADCs). A critical challenge in their development is the need for highly sensitive, specific, and high-throughput assays to accurately assess their efficacy in mediating target cell killing. Traditional cytotoxicity assays, such as chromium release, often suffer from limitations including radioactivity, high variability, and low throughput, hindering efficient drug development. The TDCC reporter assay addresses these critical gaps, providing a robust, non-radioactive, and quantitative platform essential for preclinical validation and optimization of these complex biotherapeutics, thereby accelerating their journey from concept to clinic.
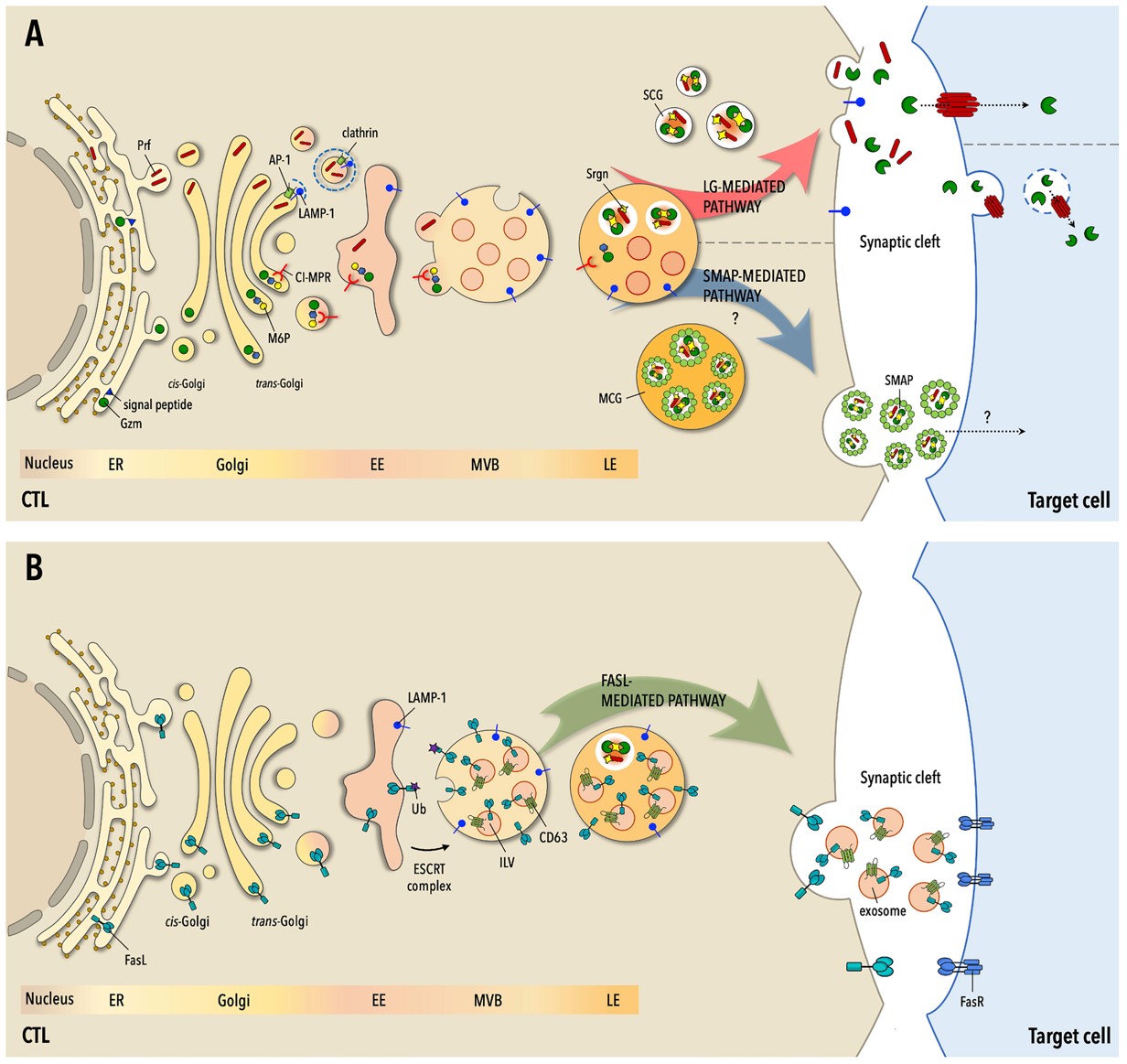 Fig.1 The three mechanisms for cytotoxic T cells (CTLs)-mediated cell death.1
Fig.1 The three mechanisms for cytotoxic T cells (CTLs)-mediated cell death.1
TDCC Reporter Assay at Creative Biolabs
Creative Biolabs' TDCC Reporter Assay provides specific and quantitative solutions for evaluating cell-mediated cytotoxicity, compound screening, antibody characterization, and functional analysis of cell therapies. By leveraging reporter gene technology, where target cells are engineered to express luciferase, we precisely measure cell viability. This principle allows for rapid, quantitative assessments, yielding high-quality, actionable data within 4 to 8 weeks to guide your drug discovery and development decisions.
Required Starting Materials
- Target Cell Lines: Specific cancer cell lines or other relevant cell types engineered to express the target antigen of interest.
- Effector Cell Lines: Immune effector cells such as T cells, NK cells, or genetically engineered immune cells (e.g., CAR-T, CAR-NK cells).
- Therapeutic Candidates: Your bispecific antibodies, ADCs, CAR constructs, or other agents to be tested.
Workflow: From Sample to Solution
Cell Line Engineering
Assay Optimization
Our experts meticulously optimize critical assay parameters, including the effector-to-target cell ratio, incubation times, and the concentration range of your therapeutic candidates, to achieve optimal sensitivity and specificity for your experimental setup.
Co-culture Setup
Engineered target cells are co-incubated with the effector cells and your therapeutic agents under precisely controlled conditions. This step mimics the in vivo cellular interactions crucial for evaluating therapeutic efficacy.
Reporter Gene Detection
Following incubation, the luminescence or fluorescence signal from the reporter gene is measured. A decrease in signal directly correlates with target cell killing, providing a quantitative measure of cytotoxicity.
Data Analysis & Reporting
Comprehensive data analysis is performed, including the generation of dose-response curves and calculation of EC50 values. You will receive a detailed experimental report with all raw data, analyzed results, and expert interpretations.
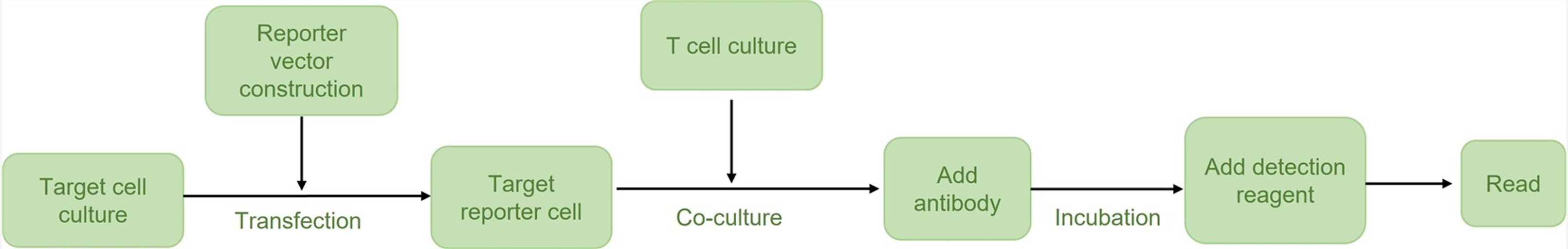 Fig.2 Assay workflow of our TDCC reporter assay.
Fig.2 Assay workflow of our TDCC reporter assay.
Service Highlights
- High Sensitivity & Specificity: Detects even subtle cytotoxic effects with minimal background interference.
- Quantitative & Reproducible: Provides accurate, measurable data, ideal for robust dose-response analysis and lead optimization.
- High-Throughput Compatibility: Designed for efficient screening of large libraries of compounds or therapeutic candidates.
- Non-Radioactive: Eliminates the safety and disposal concerns associated with traditional radioactive assays.
- Versatile Application: Adaptable to a wide range of therapeutic modalities, including bispecific antibodies, ADCs, and cell therapies.
FAQs
-
Q1: What types of therapeutic modalities can be evaluated using Creative Biolabs' TDCC Reporter Assay?
A1: Our TDCC Reporter Assay is highly versatile and can evaluate a wide range of therapeutic modalities, including bispecific antibodies, antibody-drug conjugates (ADCs), CAR-T cells, CAR-NK cells, and other immune cell engagers designed to induce target cell death. We can customize the assay to fit your specific needs.
-
Q2: How does the TDCC Reporter Assay compare to traditional cytotoxicity assays like Chromium-51 release?
A2: The TDCC Reporter Assay offers significant advantages over traditional methods like Chromium-51 release. It is non-radioactive, provides higher sensitivity and specificity, allows for high-throughput screening, and simplifies the workflow, making it a safer and more efficient alternative for cytotoxicity assessment.
-
Q3: Can Creative Biolabs customize the TDCC Reporter Assay for unique target-effector cell pairs or specific research questions?
A3: Absolutely. Our TDCC reporter assay service is highly customizable. Our expert team can work closely with you to design and optimize the assay for unique target-effector cell combinations, specific antigen expressions, and diverse therapeutic mechanisms, ensuring the assay precisely addresses your research objectives.
-
Q4: What kind of data and reports will I receive upon completion of the TDCC Reporter Assay service?
A4: Upon completion, you will receive a comprehensive report including detailed experimental protocols, raw data, analyzed dose-response curves, EC50 values, and a summary of key findings. Our reports are designed to provide clear, actionable insights to guide your drug discovery and development decisions.
Contact Us
Creative Biolabs is dedicated to supporting your scientific advancements. Reach out to us for detailed information on our TDCC Reporter Assay service or to discuss your specific project needs. Our team of experts is ready to provide personalized solutions and accelerate your research.
Reference
- Cassioli, Chiara, and Cosima T Baldari. "The Expanding Arsenal of Cytotoxic T Cells." Frontiers in immunology vol. 13 883010. 20 Apr. 2022, doi:10.3389/fimmu.2022.883010. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.
