ADCP Reporter Assay
To facilitate the development of effective cancer therapies based on monoclonal antibodies (mAbs), Creative Biolabs offers advanced ADCP reporter assays. Given the significance of antibody-dependent cell-mediated phagocytosis (ADCP) as a key mechanism of action (MOA) mediated by the antibody's Fc region, our high-quality assays provide valuable insights for clients developing innovative or biosimilar therapeutics.
Introduction to ADCP Reporter Assay
Antibody-dependent cell-mediated phagocytosis (ADCP) is a critical mechanism by which therapeutic antibodies eliminate target cells. Traditionally, assessing ADCP has been a laborious process involving primary effector cells and direct observation of phagocytosis. However, it is time-consuming and challenging. The ADCP reporter assay is a cell-based assay designed to measure antibody-dependent cell-mediated phagocytosis (ADCP) activity. Instead of directly observing phagocytosis by primary cells, this assay quantifies the activation of the intracellular signaling pathway triggered by engagement of the FcγRIIa (CD32a) receptor, providing a convenient and quantitative readout of ADCP potential.
Advantages Of ADCP Reporter Assay Over Traditional ADCP Assay
The ADCP Reporter Assay offers several advantages over traditional ADCP assays that rely on primary macrophages and direct measurement of phagocytosis:
- Reduced variability
- Simplified workflow and higher throughput
- Quantitative readout
- Elimination of primary cells
- Improved sensitivity and signal-to-noise ratio
- Cost-effective
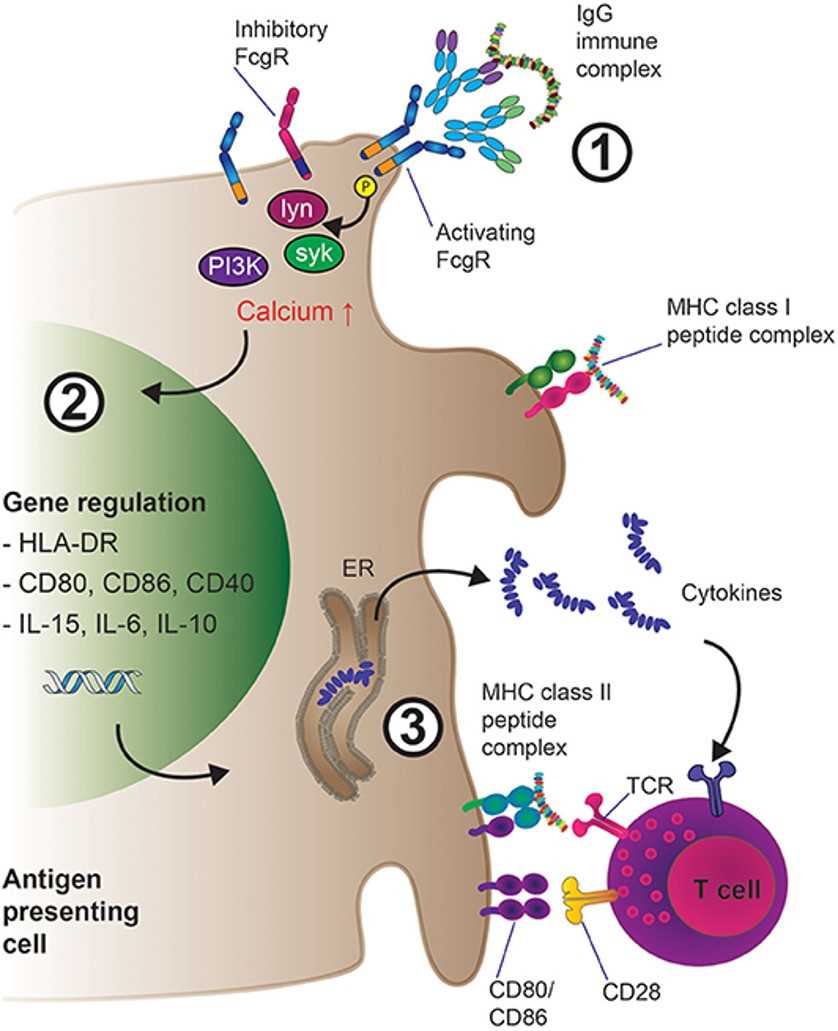 Fig.1 IgG-IG binding to low-affinity FcγRs on APCs results in increased antigen presentation and T-cell activation.1
Fig.1 IgG-IG binding to low-affinity FcγRs on APCs results in increased antigen presentation and T-cell activation.1
ADCP Reporter Assay at Creative Biolabs
Creative Biolabs offers a streamlined ADCP reporter assay utilizing an engineered Jurkat cell line stably expressing the human FcγRIIa (CD32a) H-131 variant and NFAT-luciferase. Upon engagement of a target cell-bound antibody's Fc region, this assay measures the resulting FcγRIIa signaling cascade via NFAT-driven luciferase activity, providing a readily quantifiable readout of ADCP potential. Leveraging our deep expertise in antibody effector functions and proprietary technology, Creative Biolabs delivers customizable ADCP assay solutions across various formats to support your antibody drug development throughout all stages.
Our Workflow: End-to-end Service Process
Cultured cells are transfected with reporter constructs, followed by opsonization with the investigational antibody. Post-incubation washes remove unbound immunoglobulins, after which cells are quantified and standardized to defined densities.
Phagocytes (e.g., monocytes, macrophages) are isolated or differentiated in vitro, with optional activation protocols applied. Cell counts are adjusted to achieve optimized effector-to-target ratios prior to co-culture.
Opsonized targets and effector populations are co-incubated under defined temporal and environmental conditions to permit Fc receptor-mediated engulfment.
Engulfment is assessed via luminescence assays (reporter lysis). Method-specific protocols govern signal acquisition and background correction.
Phagocytic activity is normalized against negative controls, with ADCP efficacy calculated as antibody-dependent signal differentials. Dose-response relationships and statistical significance are determined through nonlinear regression modeling and hypothesis testing.
Key Features
- Professional scientists team
- Selective reporter systems
- Customized service process
- Advanced quantification strategies
Associated Services
Beyond our ADCP reporter assay, Creative Biolabs offers a well-validated primary ADCP assay, enabling comprehensive ADCP bioactivity assessment throughout the entire lifecycle of a therapeutic antibody. Furthermore, we provide a suite of high-quality assays to quantify a wide range of other critical antibody Fc-mediated effector functions:
Q & A
-
Q1: What core elements define an ADCP reporter assay?
A1: The assay requires three principal components: antigen-presenting target cells, phagocytosis-capable effector cells, and the test antibody. A luminescent or fluorescent reporter mechanism quantifies phagocytic activity by detecting target cell engulfment.
-
Q2: Which cellular models are appropriate for experimental systems?
A2: Experimental objectives dictate target cell selection, with common models encompassing transformed cell lines, primary isolates, or pathogen-challenged cellular populations.
-
Q3: Can cell lines be used as effector cells?
A3: Indeed, their consistency and simplicity of handling make cell lines such as differentiated THP-1 quite popular.
-
Q4: Are primary effector cells better than cell lines?
A4: Comparisons of primary immune effector populations against immortalized cell models expose context-dependent trade-offs: established cell lines offer enhanced experimental consistency, but primary cells show superior physiological authenticity but exhibit donor-derived heterogeneity. Selection depends on whether the investigation goals of biological relevance or methodological repeatability take the front stage.
-
Q5: How is luminescence related to phagocytosis in luciferase-based assays?
A5: The number of target cells expressing luciferase that have lysed within the effector cells determines the luminous power released by the luciferase enzyme.
-
Q6: What factors may lead to diminished or absent ADCP activity?
A6: Potential causes might include poor antibody concentration, an unfavorable E:T ratio, improper incubation length, problems with cell viability, or the antibody's lack of usefulness in this particular test format.
For inquiries regarding our primary ADCP assays or to learn more about Creative Biolabs' comprehensive ADCP assay services, please reach out to us.
Reference
- Junker, Fabian, John Gordon, and Omar Qureshi. "Fc gamma receptors and their role in antigen uptake, presentation, and T cell activation." Frontiers in Immunology 11 (2020): 1393. Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.
