In Vitro Red Blood Cell Agglutination Assay Service
The Foundational Role and Evolving Applications of Red Blood Cell Agglutination Assays
The in vitro red blood cell (RBC) agglutination assay, or hemagglutination assay, remains a cornerstone of immunological and hematological research. Its principle is elegantly simple yet profoundly powerful: the multivalent binding of an agent—be it an antibody, a lectin, or an engineered protein—to antigens on the surface of multiple RBCs, resulting in a macroscopically visible lattice of clumped cells. This phenomenon provides a direct, visual readout of a specific molecular interaction.
Furthermore, hemagglutination assays are pivotal in drug discovery and development, particularly for screening and characterizing inhibitors of carbohydrate-binding proteins like galectins. By quantifying the inhibition of galectin induced RBC agglutination, researchers can effectively determine the potency and selectivity of small-molecule glycomimetic drugs, which hold therapeutic promise in oncology and inflammatory diseases. The assay is also a critical tool in preclinical safety assessment, used to evaluate the hemocompatibility of novel therapeutics, polymers, and biomaterials.
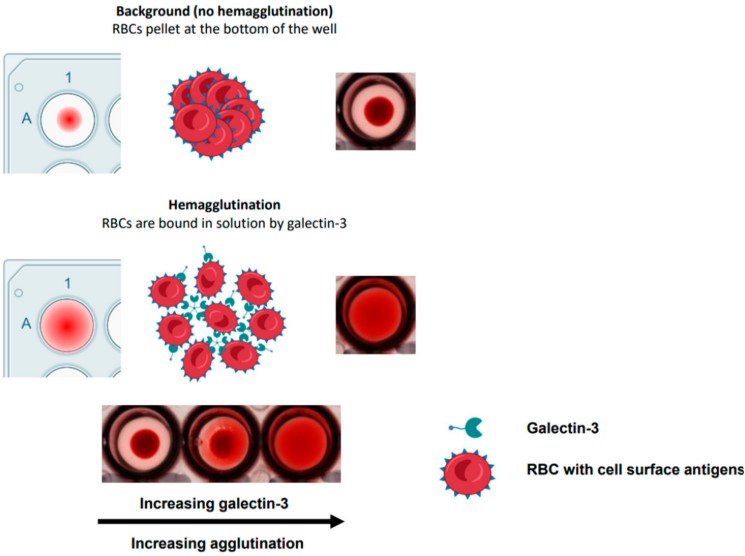 Fig.1 Galectin-3 induced hemagglutination in human red blood cells.1
Fig.1 Galectin-3 induced hemagglutination in human red blood cells.1
Our In Vitro Red Blood Cell Agglutination Assay Service
At Creative Biolabs, we have refined the classic hemagglutination assay into a robust, high-precision quantitative platform. With over two decades of specialized experience, we recognize that modern therapeutic and diagnostic development demands more than a qualitative yes/no answer. Our platform is engineered to deliver precise, reproducible, and highly granular data suitable for advanced research and regulatory submissions.
We have moved beyond simple visual inspection by integrating state-of-the-art imaging and analysis technologies. Our standard protocol utilizes U-bottom microplates, which facilitate the formation of distinct, analyzable RBC pellets or agglutination patterns. These plates are then imaged using high-resolution scanners, and the results are quantified using sophisticated image analysis software. This approach allows us to:
- Eliminate subjectivity: We replace qualitative visual scoring with objective, numerical data on the degree of agglutination.
- Enhance sensitivity: Our method can detect subtle differences in agglutination strength that are often missed by the naked eye.
- Increase throughput: The automated imaging and analysis pipeline enables efficient screening of large compound libraries or patient cohorts.
Our expertise extends to managing the complex variables that can influence assay outcomes. We have characterized the differential agglutination efficacy of agents across various blood groups (O, A, B), a critical consideration for developing therapeutics with broad applicability. We also systematically evaluate the modulatory effects of plasma proteins, as albumin and other components can significantly alter the activity of test agents, sometimes protectively, sometimes synergistically.
Comprehensive Assay Service and Deliverables
When you partner with Creative Biolabs, you gain access to a comprehensive service package designed to provide actionable insights. Our service is not a one-size-fits-all offering; it is tailored to your specific research objectives.
Our Core Service Content Includes:
- Hemagglutination / Hemolysis Screening: A primary screen to determine if a test article (e.g., antibody, lectin, polymer, small molecule) induces RBC agglutination or hemolysis.
-
Quantitative Inhibitor Characterization:
- Dose-response curve generation to determine the IC50 (half-maximal inhibitory concentration) of candidate inhibitors against a known agglutinating agent (e.g., galectin-1, galectin-3).
- Selectivity profiling against a panel of agglutinating agents to confirm target specificity.
- Blood Group Specificity Analysis: Testing the activity of a test article against a panel of RBCs from different blood groups (A, B, O) to identify any differential effects. This is crucial for understanding potential population-specific responses.
- Influence of Plasma Proteins: Assessing the impact of human serum albumin or whole plasma on the activity of the test article to provide a more physiologically relevant context for the in vitro results.
-
Full Data Package and Report:
- A detailed study protocol and methodology.
- Raw and processed quantitative data.
- High-resolution images of agglutination patterns.
- Comprehensive data analysis, including IC₅₀ calculations and statistical evaluation.
- A final, publication-quality report with a clear summary of findings and expert interpretation by our senior scientific staff.
Our Advantages
Choosing Creative Biolabs provides a distinct advantage rooted in our commitment to scientific rigor and client success.
- Unmatched Precision
- Deep Scientific Expertise
- Physiological Relevance
- Regulatory Awareness
FAQs
-
What type of samples do you accept?
We can test a wide range of purified samples, including proteins, antibodies, peptides, small molecules, and polymers. Please provide them in a suitable buffer with concentration and purity information.
-
Can you source the specific RBCs and agglutinating agents needed for my study?
Yes. Creative Biolabs maintains a supply of cryopreserved RBCs from various blood groups and can source or produce a wide array of agglutinating agents, including recombinant galectins and specific antibodies.
-
How does your quantitative method compare to standard absorbance-based readings?
Our imaging-based analysis of U-bottom plates has been shown to be superior to absorbance readings from flat-bottom plates for quantifying hemagglutination. It provides a more direct and sensitive measurement of cell clumping, avoiding artifacts from cell settling and offering a wider dynamic range.
Contact Us
Advance your research with the precision and expertise of Creative Biolabs. To discuss your project needs or to request a quote for our In Vitro Red Blood Cell Agglutination Assay services, please contact our scientific team today.
Reference
- Kruse, Robert L et al. "A rapid, point-of-care red blood cell agglutination assay detecting antibodies against SARS-CoV-2." Biochemical and Biophysical Research Communications vol. 553 (2021): 165-171. doi:10.1016/j.bbrc.2021.03.016. Distributed under an Open Access License CC BY 4.0, without modification.
For Research Use Only.
