What is DMPK?
In the long history of human resistance to disease, new drug research and development has always occupied a key position as an elite weapon for attacking tough problems. However, whether the new drugs born in the laboratory can safely and efficiently fight the disease in the human body is full of unknowns like an unopened secret letter. DMPK is like a beacon in the fog, illuminating the exploration path of new drugs from the laboratory to the clinic with the light of science - it not only decodes the absorption, metabolism and excretion trajectory of drugs in the body, but also acts like a precise navigation system to ensure that each candidate drug can find the best path to exert its efficacy in the complex physiological environment of the human body.
From aspirin turning into salicylic acid in the liver to anticancer drugs needing to break through metabolic barriers to reach the lesions, the laws of the drug's journey in the body revealed by DMPK are becoming a "safety guide" to avoid risks in new drug research and development. When scientists use DMPK data to calculate drug half-life and predict metabolic toxicity, they are essentially drawing a "human body map" for the drugs, allowing these disease-fighting chemical molecules to accurately arrive at the "battlefield" and safely exit after completing their mission, ultimately transforming the compounds in the laboratory into powerful weapons to protect health.
Two core concepts of DMPK: "drug metabolism" and "pharmacokinetics"
In the field of drug research and development in modern medicine, DMPK (drug metabolism dynamics) is a crucial concept. It is closely composed of the two core concepts of "drug metabolism" and "pharmacokinetics". Drug metabolism is like a "molecular magic show" carefully performed inside the human body. When the drug enters the human body, organs such as the liver and intestines are like precise "processing plants", where the drug will undergo a series of wonderful "disassembly" or "transformation" processes. Take aspirin, which is very familiar to us in our daily lives. When we take aspirin orally, it will go all the way along the digestive tract and eventually come to these "processing plants". Under the clever action of various enzymes, aspirin is like a magical "Transformer" and is metabolized into salicylic acid. Don't underestimate this transformation. It is salicylic acid, the "behind-the-scenes hero", that exerts its powerful effects of antipyretic, analgesic and anti-inflammatory, helping us relieve physical discomfort. It can be said that this process is like a gorgeous "transformation" of the drug in the body. Only by successfully achieving this "transformation" can the drug perform its sacred mission of curing diseases and saving lives in a more suitable form and function.
Pharmacokinetics is like a rigorous "time-concentration recorder", which focuses on exploring the subtle laws of drug concentration changes over time in the body. This process covers multiple key links such as absorption, distribution, and excretion. Drug absorption is the first step for drugs to start their journey in the body. It is about how drugs can cleverly cross layers of biological barriers and smoothly enter the huge "transportation network" of blood circulation from different routes such as entering the mouth and injection site. Just like a traveler who wants to find the way to the main road of the city, only by successfully entering the blood circulation can the drug be transported to various parts of the body. The distribution link is like a precise "courier dispatcher", which determines whether the drug can accurately reach all parts of the body, especially the most critical lesion site. Imagine that if the drug is used to treat heart disease, it must be accurately delivered to the "destination" of the heart in order to play an effective role. The excretion link is like a hard-working "cleaner", responsible for timely cleaning out the drugs and their metabolites that have completed their mission from the body to avoid excessive accumulation in the body and causing unnecessary trouble.
In short, DMPK is like an all-round "drug life historian", meticulously recording the complete life cycle of drugs from just entering ("birth") to finally being excreted ("exit"). Such detailed and accurate information is undoubtedly an indispensable and valuable asset for drug research and development. It can help scientists better understand the characteristics of drugs, optimize drug design and use plans, and thus develop safer and more effective drugs, contributing to the cause of human health.
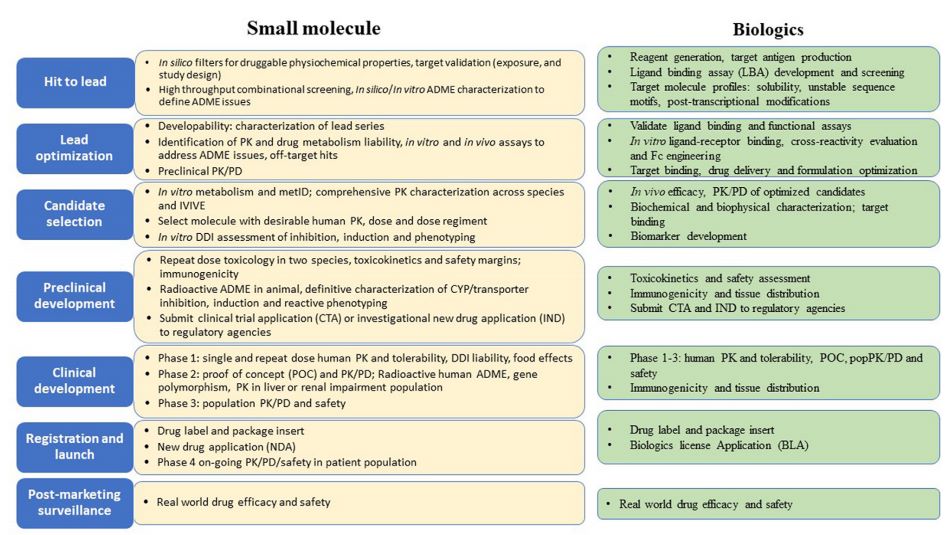 Fig. 1 Overview of DMPK related activities in drug discovery and development.1,4
Fig. 1 Overview of DMPK related activities in drug discovery and development.1,4
Why is DMPK the "life and death barrier" in new drug development?
IIn the challenging and unknown journey of drug development, the importance of DMPK (drug metabolism dynamics) is like the brightest star in the night sky, guiding the direction of research and development, and its significance is self-evident. Take the highly watched field of anticancer drug development as an example. If the research on DMPK is taken lightly, it may be like stepping into a maze full of traps and encountering many difficult difficulties.
First of all, the drug absorption link is crucial. Imagine that anticancer drugs are like a group of brave "anti-cancer warriors" who are sent to the body to fight cancer cells. However, if the drug absorption effect is not good, it is like these "warriors" are trapped in a "traffic jam", and a large number of drugs cannot be smoothly absorbed by the blood into the "battlefield". Even if these drugs have extremely powerful anti-cancer activity at the theoretical level, they cannot reach the "front line" in time and in sufficient quantities, and it is difficult to really play their due role in the body, which ultimately greatly reduces the anti-cancer efficacy, just like a carefully planned battle that fails because the troops cannot be effectively deployed. Secondly, the metabolic rate of drugs in the liver cannot be ignored. The liver is like a busy "processing plant" that processes drugs that enter the body in various ways. Some anticancer drugs are metabolized very quickly in this "processing plant", just like a car that is driving at high speed but deviates from its route. Before reaching the real "destination" of the tumor tissue, it has been broken down into pieces. In this way, these drugs cannot accurately attack cancer cells in a complete and effective form, and the goal of anti-cancer is naturally difficult to achieve. In addition, the excretion of drugs is also critical. If the drug is excreted too slowly, it is like opening a "storage warehouse" in the body, and the drugs will continue to accumulate in the body. These excessively accumulated drugs will slowly cause toxic damage to important organs such as the liver and kidneys. Once he liver and kidneys are damaged, they may cause serious adverse reactions. In this way, not only can cancer not be cured, but it will also add an extra heavy burden to the patient's body, making the already painful patients even worse.
Fortunately, the study of DMPK is like a wise "mine clearance expert" that can effectively avoid these risks. This data is like an accurate "ruler" that directly affects the frequency of drug use. If the half-life of a drug is short, it means that it disappears faster in the body. In order to maintain an effective therapeutic concentration, patients may need to take the drug multiple times a day. Conversely, if the half-life of a drug is longer, it means that it stays in the body for a relatively longer time, so the number of times the drug is taken can be appropriately reduced. In addition, scientists will also determine whether the drug metabolites are potentially toxic by analyzing the properties of the drug metabolites. This is like checking whether the "equipment" left by the "soldiers" has hidden dangers. Only by ensuring that these metabolites are safe and harmless can we avoid the heartbreaking tragedy of "curing the disease but damaging the liver", fundamentally ensure the safety of the drugs, and make anti-cancer drugs truly a reliable weapon for patients to fight the disease, rather than causing new harm.
Service you may interested in
The "dual-track model" of DMPK research: from test tube to in vivo
The research of DMPK has a rigorous whole process from laboratory to clinic. In the in vitro research stage, scientists use liver cells, intestinal cells, etc. in culture dishes to simulate the metabolic environment of drugs in the body. With the help of advanced LC-MS (liquid chromatography-mass spectrometry) technology, they can accurately detect drug metabolites and understand the metabolic pathways and changes of drugs at the cellular level. In vivo research is mainly carried out on experimental animals such as mice and rats. By injecting drugs and taking blood at regular intervals, the drug concentration at different time points is detected and a "drug concentration-time curve" is drawn. This curve is like a "track map" of the drug's journey in the animal body, clearly showing the location and concentration changes of the drug at different time points. Based on the data obtained from animal experiments, researchers further calculate the appropriate dose of the drug in the human body. Due to differences between different species, the metabolism of drugs in animals may be different from that in humans. For example, the half-life of a drug in rats is 2 hours, which may be extended to 4 hours in humans. Based on these calculation results, researchers can design a reasonable clinical trial medication plan to ensure the safety and effectiveness of clinical trials.
DMPK's "Road to Counterattack": From an Unpopular Discipline to an Industry Need
Looking back at the development of DMPK, it has gradually become a rigid demand in the field of drug research and development from a once little-known unpopular subject. Before the 1960s, the focus of drug research and development was mainly on "drug efficacy", and people paid little attention to the metabolic and kinetic properties of drugs. However, the outbreak of the thalidomide (thalidomide) incident in 1961 completely changed this situation. Due to the lack of sufficient research on the teratogenicity and metabolic properties of thalidomide, this drug caused thousands of fetal malformations, resulting in a huge tragedy. This incident sounded the alarm for global drug research and development, promoted the development of drug safety evaluation, and also made DMPK gradually come into people's vision and become a must in the drug research and development process. DMPK is like a "special tour guide" for drugs in the human body. Its existence ensures that drugs can move along the right path and work safely and effectively. In future drug development, DMPK will continue to play a key role, helping to create more innovative drugs and safeguarding human health. In the next article, we will explore the unique charm of DMPK in the drug discovery stage and see how it can use its keen insight to screen out the most promising candidate compounds.
References
- Lai, Yurong et al. "Recent advances in the translation of drug metabolism and pharmacokinetics science for drug discovery and development." Acta pharmaceutica Sinica. B vol. 12,6 (2022): 2751-2777. https://doi.org/10.1016/j.apsb.2022.03.009
- Hirabayashi, Hideki. "What is the definitive role of DMPK research for successful drug discovery and development? Until now and in the future." Drug metabolism and pharmacokinetics vol. 29,5 (2014): 357-9. https://doi.org/10.2133/dmpk.dmpk-14-pf-905
- Baillie, Thomas A. "Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism." Chemical research in toxicology vol. 21,1 (2008): 129-37. https://doi.org/10.1021/tx7002273
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
