In Vitro ADME & In Vivo PK Studies: A Holistic Framework for Drug Evaluation
Introduction to ADME/PK Studies in Drug Development
In the landscape of modern drug development, ADME (Absorption, Distribution, Metabolism, Excretion) and PK (Pharmacokinetics) studies serve as foundational pillars for evaluating candidate compounds. ADME refers to the dynamic processes governing how a drug interacts with the body, while PK quantifies the time-dependent changes in drug concentration in biological matrices. These evaluations are indispensable for reducing clinical attrition, as flawed ADME/PK properties account for up to 30% of drug failures during development. Additionally, they enable optimization of dosing regimens to achieve therapeutic efficacy and ensure compliance with regulatory standards, such as those set by the FDA and EMA.
The integration of in vitro ADME and in vivo PK analyses provides a tiered approach: in vitro models offer rapid screening of early-stage candidates, while in vivo studies bridge preclinical findings to clinical translatability. This synergy is critical for predicting human response and minimizing risks associated with off-target effects or suboptimal exposure.
In Vitro ADME Studies: Techniques and Applications
In vitro ADME assays leverage controlled experimental systems to characterize a compound's fundamental pharmacokinetic properties. Core methodologies include:
- Permeability assessment: Caco-2 cell monolayers mimic intestinal epithelium to evaluate oral absorption, while MDCK cells (Madin-Darby canine kidney) assess passive diffusion and transporter-mediated transport.
- Metabolic stability: Microsomal or hepatocyte-based assays measure a compound's susceptibility to enzymatic degradation, identifying potential liabilities like short half-life or reactive metabolite formation.
- Protein binding studies: Equilibrium dialysis or ultrafiltration techniques quantify plasma protein binding, which influences drug distribution and efficacy.
- Transporter interaction assays: Studies using transfected cell lines (e.g., expressing P-glycoprotein) evaluate potential drug-drug interactions (DDIs) by assessing substrate or inhibitor profiles.
Automated workflows, such as liquid chromatography-mass spectrometry (LC-MS/MS), enable high-throughput screening, processing hundreds of samples daily. These in vitro models offer distinct advantages: they are cost-effective compared to in vivo studies, reduce animal use, and allow species-independent testing (e.g., transgenic cell lines expressing human drug-metabolizing enzymes). Crucially, they provide predictive insights into human PK, such as estimating hepatic clearance or identifying metabolites that may pose toxicity risks.
In Vivo PK Studies: Bridging Preclinical and Clinical Data
In vivo PK studies in animal models (e.g., rodents, non-human primates) characterize a compound's behavior in a physiological context. Key parameters include:
- Biological half-life (T1/2): The time required for drug concentration to halve, guiding dosing frequency.
- Clearance (CL): The rate at which the body eliminates the drug, influencing systemic exposure.
- Bioavailability (BA): The fraction of the drug reaching the systemic circulation, critical for oral formulations.
- Tissue distribution: Imaging or bioanalytical techniques (e.g., LC-MS, ELISA) map drug accumulation in organs, informing target engagement and potential off-target effects.
Applications extend to dose optimization, where PK data inform the therapeutic window, and toxicokinetics, which link systemic exposure to adverse effects. Surgical models, such as bile duct cannulation, enable direct measurement of biliary excretion, while microdialysis probes sample extracellular fluid in real time. These studies also assess DDIs by co-administering compounds and monitoring changes in PK profiles, the foundation for clinical drug interaction studies.
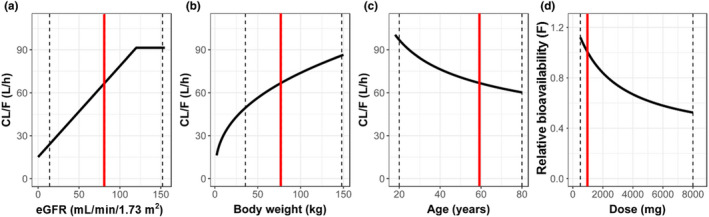 Fig.1 Influence of covariates on imeglimin pharmacokinetics. 1,2
Fig.1 Influence of covariates on imeglimin pharmacokinetics. 1,2
Comparative Analysis: In Vitro vs. In Vivo Approaches
The methodological divide between in vitro and in vivo approaches reflects their distinct scopes:
- In vitro ADME relies on reductionist models (e.g., cell monolayers, enzyme preparations) to isolate specific ADME properties. High-throughput capabilities facilitate early-stage candidate triage but may lack physiological complexity, such as inter-organ crosstalk or immune-mediated clearance.
- In vivo PK captures holistic system-level dynamics, including absorption from the gastrointestinal tract, tissue binding, and adaptive metabolic responses. However, animal studies are resource-intensive, require longitudinal sampling, and may not fully recapitulate human physiology.
Synergy between the two is essential: in vitro metabolic stability data, for example, can be integrated with in vivo PK profiles to refine human dose predictions. This integration addresses limitations—such as in vitro models' inability to account for in vivo enzyme induction or transporter saturation—by combining mechanistic insights with real-world exposure data.
Service you may interested in
ADME In Vitro-In Vivo Extrapolation (IVIVE): Principles and Tools
IVIVE aims to predict in vivo PK from in vitro parameters using physiological-based pharmacokinetic (PBPK) modeling. The process involves scaling in vitro metrics (e.g., hepatic microsomal clearance) to whole-body clearance by incorporating physiological parameters (e.g., liver blood flow, organ volumes). PBPK models simulate drug distribution across tissues, enabling predictions of human exposure without extensive animal testing.
Case studies illustrate its utility: for protein degraders, IVIVE has been used to simulate clinical PK by integrating in vitro target engagement and metabolic stability data. Similarly, reverse biokinetics—linking in vivo toxicity to in vitro metabolite formation—has aided risk assessment for compounds with reactive intermediates. Tools like physiologically based simulation software have streamlined this process, though challenges remain in accurately modeling transporter-mediated clearance and inter-individual variability.
Market Landscape of ADME/PK Studies
The global ADME/PK services market is projected to reach $10.2 billion by 2032, growing at a CAGR of 5.8%. Drivers include the rise of personalized medicine, increasing complexity of biologics (e.g., antibodies, peptides), and the need for expedited drug development. The Asia-Pacific region, particularly China, has emerged as a key growth hub, fueled by expanding pharmaceutical R&D investments and specialized in vitro ADME testing capabilities.
Technological innovation is reshaping the sector: artificial intelligence (AI)-driven platforms now predict ADME properties from chemical structure, reducing reliance on experimental screening. Automated liquid handling systems and miniaturized assays (e.g., 384-well plate formats) have enhanced throughput and cost-efficiency, while organ-on-a-chip models seek to bridge the gap between in vitro and in vivo systems.
Challenges and Emerging Innovations
Despite advancements, integration barriers persist. Scaling in vitro metabolic data to in vivo models remains problematic due to differences in enzyme expression (e.g., species-specific isoforms) and non-hepatic clearance pathways. Regulatory challenges also exist, as non-GLP (Good Laboratory Practice) in vitro assays may face scrutiny during IND (Investigational New Drug) submissions, necessitating standardized validation protocols.
Innovations are addressing these gaps: quantum AI algorithms have demonstrated 99% accuracy in predicting ADME-toxicity (ADME-Tox) profiles, outperforming traditional quantitative structure-activity relationship (QSAR) models. Robotic liquid handlers enable precise, miniaturized assays with picomolar sensitivity, while organoid cultures (e.g., liver spheroids) recapitulate tissue architecture, improving metabolic prediction. Single-cell omics technologies are also emerging, allowing characterization of cell-specific drug responses to refine PK models.
Conclusion and Future Perspectives
The integration of in vitro ADME and in vivo PK studies, anchored in IVIVE and PBPK modeling, is central to precision drug development. This approach not only enhances predictability of human response but also accelerates candidate progression while reducing animal use. Future growth will likely focus on biologics—where ADME is complicated by immunogenicity and target-mediated clearance—and AI-driven workflows that automate data analysis and modeling.
As the pharmaceutical industry shifts toward personalized and precision medicines, the ability to integrate multi-scale ADME/PK data will be indispensable. Collaborative efforts between academia, industry, and regulators to standardize methodologies and validate predictive tools will be key to realizing this vision, ensuring safer, more effective therapeutics reach patients faster.
If you want to learn more about the transgenic mice, please refer to:
- Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
- In Vitro ADME Screening: Accelerating Drug Development
- In Vitro ADME Assays: Principles, Applications, and Protocols
- Differences and Connections Between ADME and DMPK in Drug Research
- In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insight
- ADME in Toxicology: Ensuring Drug Safety and Efficacy
References
- Tomita, Yoshiko, et al. "Imeglimin population pharmacokinetics and dose adjustment predictions for renal impairment in Japanese and Western patients with type 2 diabetes." Clinical and Translational Science 15.4 (2022): 1014-1026. https://doi.org/10.1111/cts.13221
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
