Humanized Mice in Immuno-Oncology Research: Progress & Challenges
Introduction to Humanized Mouse Models
Humanized mouse models have revolutionized immuno-oncology research by providing a living platform to study human immune systems and tumor interactions in a controlled preclinical setting. At their core, these models involve the genetic, cellular, or tissue-level reconstitution of immunodeficient mice with human components, enabling researchers to mimic human immune responses to cancer.
The evolution of humanized mice has mirrored advancements in biotechnology. Early models relied on random transgenesis to express human genes, but modern approaches leverage CRISPR-Cas9 genome editing for precise engineering. This technological shift has enabled the creation of more physiologically relevant models, addressing limitations like species-specific cytokine incompatibility and incomplete immune reconstitution. Today, these models serve as indispensable tools for drug development, tumor microenvironment (TME) studies, and personalized therapy validation.
Classification & Technical Approaches
Model Types and Their Application
Humanized tumor models vary in design, each optimized for specific research needs:
- Hu-PBMC Models: These models involve transplanting human peripheral blood mononuclear cells (PBMCs) into immunodeficient mice, enabling rapid immune reconstitution within 3–4 weeks. However, they carry a risk of graft-versus-host disease (GVHD), limiting their use to short-term drug screening for acute responses.
- Hu-HSC Models: Hematopoietic stem cell (HSC)-reconstituted mice offer long-term stability (over 1 year) but exhibit reduced T-cell functionality. They are ideal for chronic disease modeling, such as persistent viral infections or slow-growing tumors.
- BLT Models: The bone marrow-liver-thymus (BLT) approach provides the most comprehensive immune reconstruction, including adaptive and innate immune cells. However, ethical concerns related to fetal tissue use restrict their broader application, though they remain critical for TME studies.
- PDX + Immune Models: Patient-derived xenograft (PDX) models combine primary tumor tissue with autologous immune cells, enabling personalized therapy prediction. These models bridge clinical relevance with preclinical feasibility, particularly for precision oncology.
Innovations in Host Engineering
Advances in host mouse engineering have enhanced model fidelity:
- Cytokine-humanized hosts: Expression of human cytokines like IL-3 and GM-CSF supports myeloid cell development, addressing a historical limitation in immune reconstitution.
- HLA-matched systems: Human leukocyte antigen (HLA) typing in hosts improves antigen presentation accuracy, crucial for testing T-cell-based therapies and immune checkpoint inhibitors (ICIs).
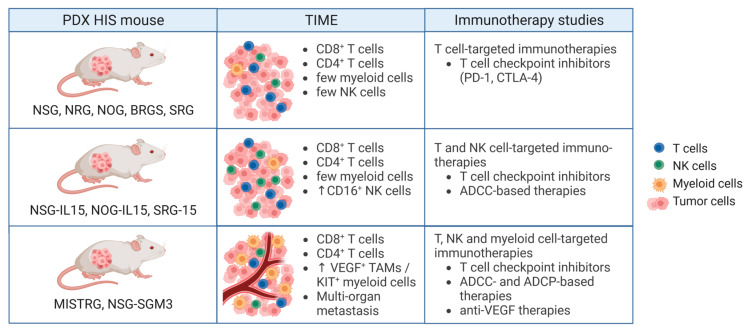 Fig.1 Humanized PDX mouse models for cancer immunotherapy.1,2
Fig.1 Humanized PDX mouse models for cancer immunotherapy.1,2
Service you may interested in
Translational Applications in Immuno-Oncology
Immune Checkpoint Inhibitor Validation
Humanized mice have been pivotal in developing ICIs, such as anti-PD-1 and anti-CTLA-4 antibodies. These models recapitulate human immune evasion mechanisms, allowing researchers to optimize dosing regimens and predict clinical responses. For example, HLA-matched models have shown correlation between PD-1 blockade and T-cell activation in solid tumors, guiding clinical trial design.
Cell Therapy Development
- CAR-T, TIL, and NK Cell Therapies: Humanized mice enable preclinical testing of cellular therapies of efficacy and toxicity. CAR-T cells targeting solid tumors, often challenging in traditional models, have shown promising activity in BLT models, while TIL therapies have demonstrated 40% tumor regression in melanoma models.
- CAR-NK Cells: Rapid PBMC-reconstituted models have facilitated CAR-NK development, showing 70% tumor clearance in colorectal cancer studies, highlighting their potential for off-the-shelf therapies.
Tumor Microenvironment Modeling
Humanized mice reveal TME complexities, such as myeloid cell-mediated immunosuppression and T-cell exhaustion. In pancreatic cancer models, combined CSF-1R/PD-1 blockade reversed T-cell suppression, underscoring the value of these models in identifying combination therapy targets.
Preclinical Case Studies: Translating Models to Mechanistic Insights
Personalized Cancer Therapy Models
- Head and Neck Squamous Cell Carcinoma (HNSCC): Autologous PBMC-PDX models with 100% HLA matching showed strong correlation between anti-PD-1 response and tumor mutational burden, guiding patient stratification for clinical trials.
- Non-Small Cell Lung Cancer (NSCLC): Hu-HSC-PDX models demonstrated synergy between anti-PD-1 therapy and chemotherapy, supporting clinical trials of combination regimens.
Blood and Solid Tumor Mechanisms
- Multiple Myeloma: The MISTRG model, engineered to express human cytokines, preserves tumor clonal heterogeneity, enabling studies of CAR-T resistance mechanisms.
- Pancreatic Cancer: BLT models revealed that CSF-1R inhibition restores T-cell activity in the fibrotic TME, leading to ongoing clinical trials of CSF-1R/PD-1 combination therapy.
Novel Therapy Platforms
| Therapy Type | Model System | Key Outcome |
|---|---|---|
| TIL Therapy | Hu-PBMC + autologous tumor | 40% tumor regression in melanoma models, validating clinical efficacy signals. |
| Bispecific Antibodies | Hu-HSC model | Complete response in B-cell lymphoma models, supporting clinical translation. |
| CAR-NK Cells | Rapid PBMC models | 70% tumor clearance in colorectal cancer, highlighting off-the-shelf potential. |
Technical Optimizations
- Myeloid cell enhancement: Cytokine-engineered hosts (e.g., for GM-CSF) improve dendritic cell recovery, enhancing antigen presentation in vaccine studies.
- Treg functional studies: SGM3 hosts (expressing human SCF, GM-CSF, and IL-3) enable detailed analysis of regulatory T cell (Treg) suppression mechanisms in cancer.
Current Challenges and Emerging Solutions
Biological Limitations
- Incomplete myeloid reconstitution: Humanized mice often lack mature myeloid cells, such as tissue-resident macrophages, limiting TME modeling.
- Cytokine cross-species incompatibility: Mouse cytokines may not fully support human immune cell development, necessitating human cytokine expression.
Technical Hurdles
- GVHD in PBMC models: Rapid T-cell activation in Hu-PBMC models causes GVHD, restricting study duration.
- Donor variability: Primary human cells from different donors yield inconsistent results, complicating reproducibility.
- Short murine lifespan: Models requiring long-term follow-up (e.g., for metastatic disease) are limited by mouse longevity.
Solutions in Development
- Purified T-cell transplants: Depleting alloreactive T cells reduces GVHD in PBMC models, extending study windows.
- Multi-donor screening: Pooling cells from multiple donors improves model reproducibility, mimicking human population diversity.
- Aging models: Genetically engineered mice with extended lifespans are being developed for chronic disease studies.
Future Directions: Advancing Model Fidelity and Translational Impact
Next-Generation Models
- iPSC-derived immune cells: Induced pluripotent stem cell (iPSC)-based models may replace fetal tissue in BLT models, addressing ethical concerns and enabling standardized, genetically defined immune reconstitution.
- Organoid-immune co-culture systems: Combining human tumor organoids with immune cells in mice could recapitulate tissue-specific TMEs, such as the intestinal or pulmonary microenvironments.
Translational Acceleration
- Microdosing clinical trials: Integrating humanized mouse data with early-phase clinical microdosing studies may bridge preclinical and clinical insights, reducing trial costs and accelerating drug approval.
Humanized mouse models have transformed immuno-oncology research, providing a critical bridge between basic science and clinical translation. From enabling ICIs to advancing cell therapies, these models continue to evolve alongside technological innovations. While challenges in immune reconstitution and translational validity persist, ongoing advancements in genome editing, iPSC technology, and personalized modeling promise to further enhance their utility. As the field progresses, humanized mice will remain indispensable for decoding tumor-immune interactions and driving the next generation of cancer therapies.
Advance Your Oncology Research with Custom Humanized Mouse Tumor Models
At Creative Biolabs, we bring years of extensive experience to the construction of humanized mouse tumor models. We understand that each research project is unique, which is why we specialize in customizing the most suitable models to meet your specific study requirements.
Ready to discuss your research needs and find the perfect model? Contact us today for a personalized consultation!
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Chen, Anna, Ines Neuwirth, and Dietmar Herndler-Brandstetter. "Modeling the tumor microenvironment and cancer immunotherapy in next-generation humanized mice." Cancers 15.11 (2023): 2989. https://doi.org/10.3390/cancers15112989
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
