Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
Transgenic mice are pivotal in cancer research, leveraging genetic plasticity via tools like CRISPR/Cre-LoxP to model oncogenic mutations with spatiotemporal control, including humanized immune-adapted models. They dissect mechanisms (e.g., oncogene activation, tumor microenvironment roles) and validate therapies: CAR-T, ADCs, vaccines, and drug resistance. As translational bridges, they inform preclinical efficacy, toxicity assessment, and personalized PDX models. Innovations like multi-gene editing, organoid-chimeras, and real-time imaging enhance precision. These models offer end-to-end support from mechanistic insights to therapeutic development, accelerating cancer research through controllable genetics and human-like physiology.
The Central Role of Transgenic Mice in Cancer Research
Transgenic mice are central to cancer research, leveraging 80% genetic homology with humans and advanced gene editing. Shared oncogenes (e.g., MYC) and tumor suppressors (TP53) ensure faithful mimicry of human cancer biology, drug responses, and mechanisms like mutation-driven transformation and immune evasion. CRISPR/Cas9 enables precise, spatiotemporal gene manipulation (e.g., tissue-specific cancer induction), while viral vectors create models with clinical features (e.g., liver cancer via hepatitis B genes). These tools dissect tumor microenvironments and treatment resistance, accelerating translation from basic research to clinical applications like targeted drug testing. Genetic conservation and technical precision make them indispensable for understanding cancer mechanisms and advancing therapies.
Transgenic technology breakthroughs, led by CRISPR/Cas9 and viral vectors, are pivotal in cancer research. CRISPR enables precise genome editing—targeting oncogenes/tumor suppressors and spatiotemporal control (e.g., tissue-specific KRAS activation in lung cancer models to study mutation-driven growth and microenvironment interactions). Viral vectors efficiently introduce foreign genes (e.g., HBV's HBx) to model virus-associated cancers like liver cancer, replicating histology, metastasis, and chemoresistance for drug sensitivity testing. These tools create high-fidelity models of tumor heterogeneity, invasiveness, and treatment responses (e.g., EGFR-driven resistance), accelerating screening of targeted/immunotherapies and informing combination strategies. By bridging mechanistic precision with clinical phenotype mimicry, they drive translational research from basic discovery to personalized therapy validation.
Tumor Model Development: From Seeding Mechanisms to Complex Systems
Tumor Seeding Types
Cancer mouse models employ various tumor transplantation methods, each tailored to specific research goals and differing in tumor microenvironment simulation, research focus, and clinical relevance:
- Subcutaneous injection involves inoculating cancer cell suspensions into the mouse flank, forming visible tumors. Its simplicity and low cost enable real-time growth monitoring, making it ideal for preliminary chemotherapy/targeted drug screening and angiogenesis studies. However, it lacks physiological microenvironment interaction, minimal metastasis, and limited clinical translation due to simplified tumor biology.
- Orthotopic transplantation implants cells/tissues into their native organ sites (e.g., mammary fat pads, pancreas), replicating the primary tumor microenvironment. This method models tumor-stromal/immune cell interactions, suitable for studying invasion, organ-specific metastasis (e.g., breast cancer lung metastasis), and microenvironment effects on therapy, with high clinical relevance. Drawbacks include technical complexity (surgery/imaging guidance) and difficult growth monitoring.
- Intravenous injection via the tail vein mimics hematogenous metastasis, studying circulating tumor cell (CTC) colonization in distant organs (lungs, liver) and anti-metastatic drug efficacy. While directly modeling blood dissemination, lesion distribution is random and hard to control, fitting specific metastatic mechanism exploration.
- Intraperitoneal injection targets peritoneal dissemination (ovarian/gastric cancer), creating ascites and peritoneal tumor interactions, useful for intraperitoneal chemotherapy permeability testing. It simulates extensive peritoneal metastasis but complicates evaluation due to uneven lesion distribution and mixed local/systemic drug effects.
- Lymph node injection focuses on early lymphatic metastasis, investigating cancer cell migration through lymphatics and tumor-immune cell (T cells, dendritic cells) interactions in lymph nodes. It captures initial metastatic events but requires high technical precision for stable model establishment.
Selection depends on research objectives: subcutaneous injection for simple local growth screening; orthotopic transplantation for microenvironment/metastasis studies; and intravenous/intraperitoneal/lymph node injection for specific metastatic pathways. Each method balances mechanistic depth and clinical translation potential, requiring alignment of model strengths with research needs to bridge basic science and application effectively.
Syngeneic Models vs. Xenograft Models
In cancer research, the choice between syngeneic and xenograft models hinges on study objectives, balancing immune system functionality with clinical relevance to human tumors.
Syngeneic models utilize tumor cells genetically matched to immunocompetent mouse strains (e.g., B16 melanoma in C57BL/6 mice), preserving intact immune systems. This allows robust investigation of immune-oncology therapies like checkpoint inhibitors (e.g., anti-PD-1) and CAR-T cells, as they recapitulate T cell-mediated tumor killing and microenvironmental interactions—such as T cell infiltration and immunosuppressive pathways. For example, these models effectively assess how PD-1/PD-L1 blockade activates antitumor immunity, mirroring human immune responses. However, their limitation lies in reliance on murine tumor cells, which cannot directly model human-specific tumor antigens.
Xenograft models involve implanting human tumor cells/tissues into immunodeficient mice (e.g., NSG mice), creating an immune-isolated environment that enables human tumor growth without rejection. Ideal for chemotherapy and targeted therapy screening (e.g., EGFR inhibitors, antibody-drug conjugates), they directly evaluate drug efficacy on human-derived tumors, capturing biological features like angiogenesis and drug metabolism more clinically relevant to humans. For instance, they assess sensitivity of human lung cancer cells to tyrosine kinase inhibitors. Yet, their lack of functional T/B cells precludes studies of immune-dependent therapies, and disparities in stromal cell interactions may affect tumor behavior.
Model selection is guided by research goals: syngeneic models dominate immune therapy studies due to intact immune-tumor crosstalk, while xenografts excel in non-immunotherapeutic contexts, prioritizing human tumor biology. Researchers must weigh the need for immune system fidelity against the requirement for direct human tumor representation, ensuring alignment with mechanistic hypotheses and translation.
A comparative analysis of syngeneic and xenograft models (Table 1).
| Feature | Syngeneic Models | Xenograft Models |
| Immune Status | Immunocompetent | Immunodeficient (e.g., NSG mice) |
| Tumor Source | Mouse-derived cell lines | Human tumor cells/tissues |
| Applications | Immunotherapy (e.g., anti-PD-1) | Chemotherapy screening |
Humanized Mouse-Based Tumor Models
To bridge the gap between xenograft and syngeneic models, humanized mouse models have been developed to reconstitute aspects of the human immune system in mice. There are two primary approaches:
- Hu-PBMC Models: Hu-PBMC models involve the engraftment of human peripheral blood mononuclear cells (PBMCs) into immunodeficient mice, providing a rapid means to assess T-cell function and antigen-specific responses. However, these models are prone to GvHD, as human T cells may recognize murine antigens, limiting their long-term utility.
- Hu-HSC Models: Hu-HPSC models address this limitation by transplanting human hematopoietic stem cells (HPSCs) into irradiated mice, allowing for the de novo development of a human immune system, including T cells, B cells, and myeloid cells. This approach enables long-term studies of immune-tumor interactions, such as the efficacy of CAR-T cell therapies over extended treatment periods.
Technical refinements, such as the development of NOG-FcγR KO mice—which lack murine Fcγ receptors to eliminate antibody-mediated interference—have enhanced the accuracy of antibody therapy evaluation in these models. These advancements have made humanized mice indispensable for preclinical trials of immune-based therapies.
Therapeutic Development: Key Efficacy Evaluation Strategies
The development of effective cancer therapies relies heavily on rigorous preclinical evaluation using appropriate animal models. Transgenic mice play a crucial role in assessing the efficacy of various therapeutic modalities.
Antibody-Drug Conjugates (ADCs)
ADCs represent a class of targeted therapies that combine monoclonal antibodies with cytotoxic payloads, designed to deliver drugs specifically to tumor cells. Evaluation involves:
- In Vitro: Cytotoxicity assays are employed to determine the ADC's ability to kill cancer cells, while antigen affinity assays measure the strength of the antibody-target interaction. Bystander effect analysis assesses the ADC's capacity to eliminate neighboring, non-targeted cells upon payload release.
- In Vivo: Pharmacokinetic (PK) studies in CDX and PDX models are conducted to understand the ADC's behavior within a living organism, including its absorption, distribution, metabolism, and excretion. Tumor suppression studies in these models evaluate the ADC's ability to inhibit tumor growth.
Clinical translation of ADCs relies heavily on these models to predict the balance between efficacy and toxicity, particularly for payloads with narrow therapeutic indices, such as maytansinoids and auristatins.
CAR-T and CAR-NK Cell Therapies
Transgenic mice, as a key experimental platform for the development of CAR-T/NK cell therapy, play a core role in target validation, efficacy evaluation, and toxicity prediction. Among them, the xenograft model (human tumor-immunodeficient mouse) can accurately evaluate the targeting of treatment by simplifying the system to eliminate host immune interference, such as verifying the ability of CD19-CAR-T to specifically eliminate B cell leukemia cells, while monitoring off-target toxicity to protect normal tissues; while the humanized immune system (HIS) model focuses on simulating clinical-grade immune responses and deeply analyzing treatment side effects, such as exploring the mechanisms of cytokine release syndrome CRS and neurotoxicity, and can also conduct research from the dimensions of interaction between CAR-T cells and the human immune microenvironment and dynamic exhaustion of immune cells.
However, current technologies face many challenges. Complex phenotypic monitoring urgently needs to achieve high-precision detection of dynamic changes in cytokines in the tumor microenvironment and real-time tracking of T cell exhaustion markers, which relies on technologies such as multicolor flow cytometry and in vivo imaging. The model itself also has limitations. The HIS model is difficult to fully reproduce human immune diversity, and the transplant model is relatively simplified in presenting tumor heterogeneity, which affects the accuracy of clinical prediction.
In the future, technological development will focus on the integration of multimodal monitoring technologies, using spatial transcriptome and single-cell sequencing to analyze the immune cell-tumor cell interaction network, and using high-dimensional flow cytometry and in vivo imaging to track treatment response and toxicity events; model optimization is moving towards the construction of a more complete human immune component and the development of a "patient-derived HIS model". Through the division of labor and cooperation between xenograft models and HIS models, a technical closed loop from target screening, efficacy/toxicity evaluation, mechanism mining to clinical transformation is formed, and finally the ultimate goal of optimizing the safety and effectiveness of CAR-T/NK therapy and accelerating the clinical implementation of precision immunotherapy is achieved.
Oncolytic Viruses
Oncolytic viruses (OVs) act as a "double-strike weapon": genetically programmed to selectively replicate in and lyse tumor cells directly, while releasing tumor antigens and danger signals (DAMPs) to recruit CD8+ T/NK cells and trigger IFN-γ-driven immune responses against residual cancer cells. Researchers use a three-level evaluation system: qPCR/immunofluorescence for viral replication, in vitro/in vivo assays for oncolytic efficacy, and immune cell infiltration/IFN-γ levels to assess immune activation. In combination therapy, OVs synergize with anti-4-1BB antibodies to boost T cell killing/memory and convert "cold tumors" to "hot tumors" with PD-1/CTLA-4 inhibitors, enhancing immune recognition. Genetically edited models (e.g., PD-1-deficient mice) simulate tumor microenvironments, enabling precise optimization of OV-immunotherapy dose/timing. This integrated approach—from mechanistic dual action to quantitative evaluation and combinatorial strategies—drives OV therapy from theoretical design to clinical translation, marking a breakthrough in cancer treatment.
- Direct Killing: Viral replication efficiency and tumor lysis in syngeneic models.
- Immune Activation: CD8+ T/NK cell infiltration and IFN-γ release in immunocompetent mice.
- Combination Strategies: Pairing with anti-4-1BB antibodies enhances antitumor immunity by activating costimulatory pathways.
Cancer Vaccines
The R&D of cancer vaccines is a sophisticated life project, aiming to activate the immune system's "precision strike" on tumor cells. The key is screening personalized tumor-specific antigens from somatic mutation-derived new antigens. Transgenic mouse models expressing human MHC molecules and antigen-presenting proteins serve as a "simulated battlefield" for vaccine design by mimicking human antigen presentation. Efficacy evaluation uses both short-term indicators (e.g., CD8+ T cell proportion in tumors) and long-term clinical outcomes (e.g., recurrence-free survival), with tumor-infiltrating lymphocytes (TILs) as a critical "ruler" for anti-tumor immunity. The Pmel model, carrying gp100-specific T cells, revealed mechanisms of enhanced T cell activation and memory, translating lab findings into metastatic melanoma vaccine trials. This process forms a closed-loop from antigen selection, model construction, effect verification to clinical application, driving cancer vaccines from theory to practice and embodying hope in cancer combat.
- Antigen Selection: Neoantigens validated via exome sequencing are prioritized for personalized vaccines.
- Model Optimization: Humanized MHC&TAP mice improve antigen presentation fidelity by expressing human leukocyte antigen (HLA) molecules.
- Efficacy Markers: Tumor-infiltrating lymphocyte (TIL) ratios and recurrence-free survival (RFS) correlate with vaccine success.
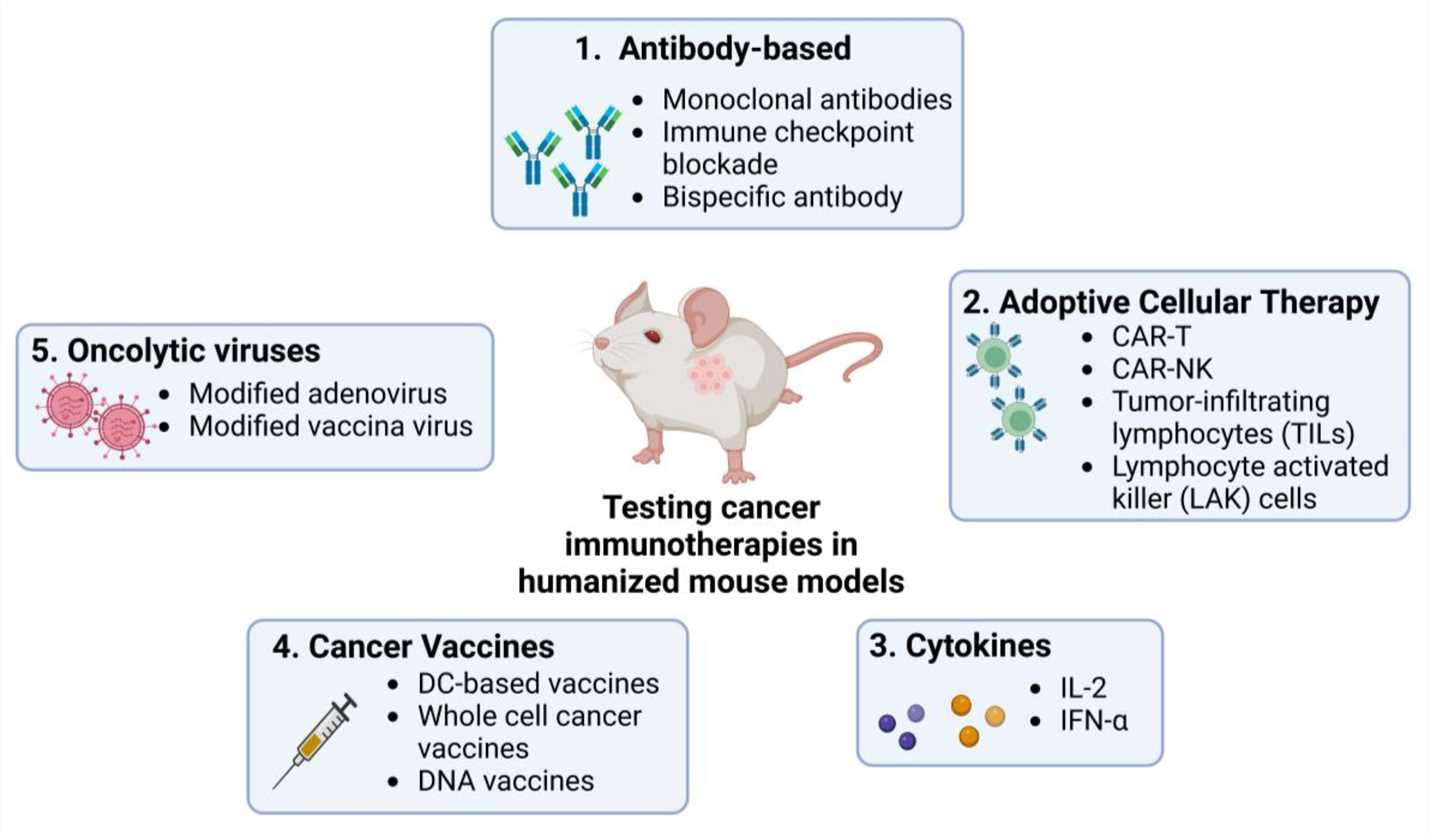 Fig. 1 Utilizing humanized mouse models to evaluate the application of current immunotherapies in cancer research. 1,2
Fig. 1 Utilizing humanized mouse models to evaluate the application of current immunotherapies in cancer research. 1,2
Service you may interested in
Classic Transgenic Mouse Strains and Their Applications
Several classic transgenic mouse strains have significantly contributed to our understanding of cancer and the development of therapies.
MMTV Transgenic Mice
The MMTV-PyMT transgenic mouse model serves as a "living laboratory" in breast cancer research, leveraging MMTV promoter-driven PyMT expression in mammary epithelial cells to induce highly penetrant breast tumors within 6 weeks, mimicking the aggressive, early-metastatic phenotype of human triple-negative breast cancer (TNBC). This model has been pivotal for mechanistic studies, uncovering core PI3K/AKT and RAS/MAPK signaling networks in tumor progression and acting as a "dynamic sandbox" to dissect metastasis cascades and tumor-microenvironment interactions. In drug development, its tumor microenvironment heterogeneity—despite lacking HER2 expression—makes it ideal for modeling drug resistance, facilitating screening of HER2 inhibitors and preclinical evaluation of combinatorial therapies (chemotherapy, immunotherapy, targeted agents). Valued for its predictability (clear tumorigenesis timeline) and traceability, it accelerates efficacy assessment and mechanism analysis but requires integration with other models to address limitations like target-specific gaps. From basic research to translational medicine, this model bridges lab discoveries and clinical applications, driving breakthroughs in TNBC understanding and therapy development.
RIP-Tag Transgenic Mice
The RIP-Tag transgenic mouse model acts as a "dual-function microscope" for tumor immunology and autoimmune disease research. Using the insulin promoter (RIP) to drive SV40 large T antigen expression in pancreatic β cells, it mimics human insulinoma, forming a "living reference system" for pancreatic endocrine tumor mechanisms. Its innovation lies in a "two-way research scenario": embryonic antigen exposure induces thymic deletion of SV40-specific T cells, establishing immune tolerance, while adult-induced antigen expression triggers spontaneous insulitis, modeling type 1 diabetes' β-cell autoattack. This spatiotemporally controlled antigen expression reveals immune tolerance establishment/breakdown mechanisms. In tumor-immune studies, it serves as an "immune regulation sandbox," uncovering tumor immune evasion via embryonic antigen exposure and evaluating tolerance-breaking therapies like dendritic cell vaccines. Valued for "dual pathological simulation" (tumorigenesis and autoimmunity) and controllable timing, it enables dynamic tolerance analysis but requires endogenous antigen models for conclusion validation. Bridging tumor immunology and diabetes research, the RIP-Tag model offers unique insights into immune-tumor interactions and translational potential.
Pmel Transgenic Mice
The Pmel transgenic mouse model serves as a "precision targeting navigator" for melanoma immunotherapy, engineering CD8+ T cells to recognize gp100, a melanoma-specific antigen, creating a standardized system to analyze anti-tumor T cell responses. In therapy development, it optimizes adoptive T cell therapy (e.g., IL-2 pre-stimulation) and evaluates synergies with checkpoint inhibitors like anti-CTLA-4, enhancing anti-tumor efficacy. Mechanistic studies using Pmel reveal long-term immune response regulators and resistance mechanisms, such as tumor antigen loss and PD-1 overexpression, driving strategies like anti-PD-1 therapy. Its translational advantage lies in clear antigen-receptor pairing, accelerating clinical translation—CTLA-4 inhibitors benefited from its validation. Though limited by single-antigen dependence, the Pmel model remains pivotal, offering an irreplaceable platform for T cell response analysis and combinatorial strategy exploration in melanoma immunotherapy.
| Strain Name | Key Oncogene/Antigen | Tissue Specificity (Promoter) | Resulting Tumor Type(s) | Notable Biological Features | Primary Research Applications |
|---|---|---|---|---|---|
| MMTV | PyMT | Mouse Mammary Tumor Virus | Mammary adenocarcinoma | High penetrance, lung metastasis | Breast cancer pathogenesis, drug screening (HER2 inhibitors) |
| RIP-Tag | SV40 T antigen | Rat Insulin Promoter | Pancreatic β-cell tumor (insulinoma) | Multistage tumorigenesis, angiogenesis, immune tolerance/insulitis | Pancreatic cancer development, tumor-immune interactions |
| Pmel | gp100 antigen-specific TCR | Endogenous TCR promoter | None (T cells recognize gp100) | CD8+ T cell recognition of melanoma antigen | Melanoma immunotherapy, adoptive T-cell transfer |
Challenges and Future Directions
In cancer research, mouse models face critical challenges: humanized models lack full human immune components (e.g., dendritic cells, mucosal immunity), limiting immunotherapy prediction; PDX models retain tumor heterogeneity but are costly and slow, unsuitable for high-throughput screening; and tumor microenvironment (TME) simulation fails to replicate stromal interactions, extracellular matrix, and vascular structures, creating a translational gap between preclinical data and human physiology.
Future breakthroughs lie in multi-omics integration (single-cell sequencing, spatial transcriptomics to map TME networks), organoid-mouse chimerism to model tissue-specific carcinogenesis, and AI-driven machine learning to predict human responses from mouse data, accelerating drug screening. These technologies address immune, TME, and throughput limitations, aiming to build "next-generation models" that bridge "simplified simulation" and "high-fidelity human substitutes," enhancing precision medicine and translational research by aligning model characteristics with human tumor physiology.
Conclusion
Transgenic mice, enabled by precise gene editing, are core models for mimicking tumor mechanisms and drug responses, bridging basic research and clinical translation. They aid in dissecting biology, accelerating therapy development, and integrating TME complexity. Challenges include cross-species immune gaps, TME replication limits, and personalized therapy adaptation. Technological advances like CRISPR optimization and multi-omics aim to refine models for human tumor mimicry. In precision medicine, they remain indispensable for cancer mechanism research and therapeutic innovation.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Custom Mouse Tumor Models for Your Research Needs
If you are looking to build mouse tumor models that better meet your experimental requirements, please get in touch with our experts. We are dedicated to customizing unique models for you. Our team of professionals has extensive experience and expertise in creating tailor - made mouse tumor models, ensuring they align perfectly with your specific research needs. Don't miss the opportunity to have models that can enhance the accuracy and success of your experiments. Contact us now to start the consultation process!
References
- Karnik, Isha, et al. "Emerging preclinical applications of humanized mouse models in the discovery and validation of novel immunotherapeutics and their mechanisms of action for improved cancer treatment." Pharmaceutics 15.6 (2023): 1600. https://doi.org/10.3390/pharmaceutics15061600
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
