Humanized Mice in Vaccine Research: From Concept to Clinical Trials
Vaccine development stands as one of the most critical frontiers in modern medicine, yet it is fraught with significant challenges. At the heart of these challenges lies the need to bridge the gap between preclinical research and human clinical trials effectively. Traditional animal models, while invaluable, often fail to accurately recapitulate human immune responses, leading to discrepancies that can hinder the translation of promising vaccine candidates into clinical applications. This is where humanized mice have emerged as a revolutionary tool, offering unprecedented opportunities to model human biology and immunology in a controlled preclinical setting.
The Technological Principles and Classification of Humanized Mice
The Challenge of Vaccine Development
The quest for effective and safe vaccines has never been more urgent, particularly in the face of emerging infectious diseases and global pandemics. Traditional animal models, such as wild-type mice or non-human primates, have long been the cornerstone of preclinical vaccine research. However, they suffer from fundamental limitations in predicting human immune responses. Species-specific differences in immune system architecture, pathogen receptor expression, and cytokine signaling often result in outcomes that do not translate to humans, leading to costly failures in clinical trials.
The Emergence of Humanized Mice
Humanized mice are defined as immunodeficient mice engrafted with human cells, tissues, or genes, designed to mimic human biology. This innovative approach serves as a vital bridge between in vitro studies and human clinical trials, offering a dynamic in vivo system to study human-specific immune responses and pathogen interactions. By reconstituting human immune components in a murine host, scientists can observe how the human immune system responds to vaccine candidates in a manner that closely resembles natural human physiology.
Scope of Humanized Mouse Applications
This article explores the multifaceted role of humanized mice in vaccine research, tracing the journey from early conceptualizations to their current use in informing critical clinical trial designs. The focus spans across technical classifications, application scenarios, case studies in COVID-19 research, and future prospects, highlighting their transformative impact on modern vaccine development.
Understanding Humanized Mouse Models for Vaccine Research
Types of Humanized Mouse Models
Human Hematopoietic Stem Cell (HSC)-Reconstituted Mice (Hu-HSC Mice)
Hu-HSC mice are generated by engrafting human CD34+ hematopoietic stem cells into immunodeficient mice. This process enables the development of diverse human immune cell lineages, including T cells, B cells, natural killer (NK) cells, and myeloid cells. The key advantages of Hu-HSC mice include long-term engraftment and de novo immune system development, making them ideal for studying primary immune responses to novel vaccine antigens over extended periods.
Human Peripheral Blood Leukocyte (PBL)-Reconstituted Mice (Hu-PBL Mice)
Hu-PBL mice are reconstituted with human peripheral blood mononuclear cells (PBMCs), resulting in a primarily T-cell-rich immune system. This model offers rapid reconstitution, making it suitable for studies requiring quick turnaround times. Hu-PBL mice are particularly useful for analyzing recall responses and pre-existing immunity, such as evaluating booster vaccine efficacy in individuals with prior pathogen exposure.
Specific Gene-Edited Humanized Mice
Gene-edited humanized mice express specific human genes, such as the angiotensin-converting enzyme 2 (ACE2) receptor for SARS-CoV-2 studies. This targeted approach allows researchers to investigate host-pathogen interactions with precision, overcoming species-specific barriers that limit studies in wild-type mice.
Advantages in Vaccine Research
Recapitulation of Human Immune Responses
Humanized mice excel in mimicking both cellular and humoral immune responses observed in humans. They facilitate the development of antigen-specific human T and B cells, as well as antibody production, providing a comprehensive platform to assess vaccine immunogenicity.
Study of Human-Tropic Pathogens
These models enable in vivo studies of pathogens that exclusively infect humans, such as HIV and SARS-CoV-2. By reconstituting human-specific receptors and immune components, researchers can observe viral replication, pathogenesis, and immune evasion mechanisms in a human-relevant context.
Evaluation of Vaccine Efficacy
Humanized mice allow for rigorous assessment of vaccine candidates' ability to elicit desired immune responses, including neutralizing antibody titers and T-cell-mediated immunity. They also enable testing of protective efficacy against pathogen challenge, providing critical data on vaccine potency.
Safety and Toxicity Profiling
These models play a pivotal role in identifying potential adverse effects of vaccine candidates on human immune cells, helping to prioritize safe candidates for clinical translation and minimizing risks in human trials.
Service you may interested in
Core Application Scenarios in Vaccine Development
Target Validation and Vaccine Design
Humanized mice with engrafted human receptors, such as ACE2, enable the validation of viral entry mechanisms. This facilitates the screening of vaccine candidate molecules targeting human-specific antigens, such as the SARS-CoV-2 spike protein, ensuring they interact effectively with human cellular receptors.
Immunogenicity and Efficacy Assessment
Humoral Immunity
Assays such as ELISA are used to measure neutralizing antibody titers and levels of human IgG/IgM, providing quantitative data on antibody-mediated immune responses.
Cellular Immunity
Flow cytometry is employed to evaluate T cell subsets (CD4+ and CD8+), cytokine secretion, and memory responses, offering insights into cell-mediated immunity critical for viral clearance and long-term protection.
Case Study
A peptide vaccine in preclinical development was shown to induce broad-spectrum T cell responses in humanized CD34+ mice, mirroring immune profiles observed in recovered patients.
Virus Challenge and Protective Efficacy Testing
Humanized mice allow for the simulation of human infection pathology, such as pulmonary immune damage in COVID-19. They also enable the validation of vaccine protection against lethal doses of pathogens, as demonstrated by a 100% survival rate in mice expressing human DPP4 challenged with MERS virus.
Vaccine Development for Special Pathogens
For pathogens like EBV and HIV, humanized mice overcome species restrictions, enabling the evaluation of vaccine-induced protection against B cell infections. They have also facilitated the development of the first small-animal model for variola virus challenge, accelerating the testing of medical countermeasures.
Typical Case Studies in COVID-19 Vaccine Research
The Critical Role of Humanized ACE2 Models
Wild-type mice express an ACE2 receptor that differs from the human variant, limiting their utility in SARS-CoV-2 research. Humanized ACE2 mice accurately model viral infection, even recapitulating age-related severe disease risk, with enhanced inflammatory responses observed in older mice, mirroring human clinical observations.
Immune System Humanized Models (e.g., DRAGA Mice)
Models expressing human leukocyte antigens (HLA), ACE2, and TMPRSS2 support SARS-CoV-2 replication and exhibit human-like pulmonary pathology, including T cell infiltration, microthrombi formation, and antibody production, providing a comprehensive platform for vaccine testing.
Vaccine Testing Outcomes
A multi-epitope peptide vaccine demonstrated the ability to activate poly-specific T cell responses in humanized mice, with immune profiles overlapping with those of recovered COVID-19 patients, validating its potential as a broad-spectrum vaccine candidate.
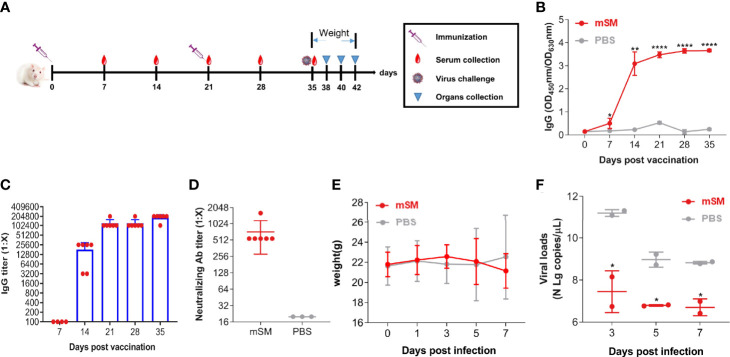 Fig.1 Assessing SARS-CoV-2 VLP immune effects in humanized mice.1,2
Fig.1 Assessing SARS-CoV-2 VLP immune effects in humanized mice.1,2
Clinical Translation Challenges and Optimization Directions
Existing Limitations
Model Deficiencies
Current models often suffer from insufficient myeloid and NK cell development, as well as lacking secondary lymphoid structures. HLA mismatches can also lead to biased immune responses, limiting translational accuracy.
Technical Bottlenecks
The production of humanized mice is associated with long preparation cycles (12 weeks for HSC models), high costs, and variable engraftment rates. Cross-species cytokine signaling incompatibilities further complicate immune system reconstitution.
Optimization Strategies
Genetic Engineering Improvements
Introducing human cytokine genes (e.g., IL-6, GM-CSF) supports more robust immune cell development, while constructing HLA transgenic mice enhances antigen presentation efficiency, improving model fidelity.
Standardization Initiatives
Establishing comprehensive model quality evaluation systems, including engraftment rate and immune cell abundance thresholds, is crucial for ensuring reproducibility and comparability across research laboratories.
Clinical Relevance Validation
Advanced approaches, such as "Avatar" models that match patient-derived tumors and immune cells, hold promise for personalized vaccine development. Shortening model construction cycles will further support real-time translational research for emerging pathogens.
Future Perspectives
Next-Generation Model Development
Integrating organoid technology with humanized mice, such as lung organoid transplantation, represents a frontier in modeling tissue-specific immune responses and pathogen interactions with unprecedented precision.
Precision Vaccine Design
Humanized mice will enable the screening of race-broad epitopes, facilitating the development of vaccines compatible with HLA polymorphism, a key factor in ensuring universal immune protection across diverse populations.
Regulatory Standardization
Advocating for standardized preclinical research guidelines will help narrow the gap between animal experiments and clinical trials, streamlining the vaccine development pipeline and accelerating the delivery of life-saving vaccines to those in need.
In conclusion, humanized mice have revolutionized vaccine research by providing a bridge between preclinical science and human clinical reality. As these models continue to evolve, they hold the promise of accelerating the development of safe and effective vaccines for a wide range of infectious diseases, ultimately improving global public health outcomes.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Chen, Jing, et al. "Immunogenicity and protective potential of chimeric virus-like particles containing SARS-CoV-2 spike and H5N1 matrix 1 proteins." Frontiers in Cellular and Infection Microbiology 12 (2022): 967493. https://doi.org/10.3389/fcimb.2022.967493
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
