In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insights
Introduction to In Vitro ADME Profiling Services
In vitro ADME (Absorption, Distribution, Metabolism, and Excretion) profiling has emerged as a cornerstone of modern drug discovery, offering predictive insights into the pharmacokinetic (PK) behavior of drug candidates long before they enter clinical trials. By simulating biological processes in controlled laboratory settings, these services enable researchers to evaluate how a compound is absorbed into the bloodstream, distributed to target tissues, metabolized by enzymes, and excreted from the body. Such evaluations are critical for identifying promising candidates early in development while discarding those with unfavorable properties, thereby reducing the risk of costly late-stage failures.
The importance of in vitro ADME profiling extends beyond scientific validation. It plays a pivotal role in optimizing resource allocation, as early-stage data helps prioritize candidates with higher chances of success. Additionally, regulatory agencies increasingly require robust ADME datasets to support Investigational New Drug (IND) and New Drug Application (NDA) submissions, ensuring that only safe and effective therapies progress to human trials. By integrating predictive models and advanced assays, in vitro ADME services bridge the gap between preclinical research and clinical translation, fostering a more efficient and sustainable drug development pipeline.
Service you may interested in
Core Components of ADME Properties
The four core components of ADME characteristics together form the framework for evaluating drug behavior in vivo. The absorption process lays the foundation for oral availability of drugs through solubility and permeability analysis, while models such as PAMPA and Caco-2 provide key data for dosage form optimization. Distribution studies focus on the dynamic distribution of drugs in blood and tissues. Protein binding rate measurement combined with tissue distribution models can reveal the effective concentration and potential accumulation risks of drugs. Metabolic analysis focuses on the liver CYP450 enzyme system and helps avoid safety hazards after exposure in the body by identifying metabolic pathways and toxic metabolites. Excretion studies clarify the laws of drug elimination by exploring the renal and bile clearance mechanisms, and provide a basis for clinical dose regimen design and drug interaction risk assessment. These four components are interconnected and together provide systematic guidance for the early screening and optimization of drug candidate compounds, ensuring that drug candidates have ideal pharmacokinetic characteristics before entering the clinic.
| Component | Key Factors & Methods | Relevance |
|---|---|---|
| Absorption | Solubility, permeability (e.g., PAMPA, Caco-2 assays) | Predicts oral bioavailability and informs formulation strategies. |
| Distribution | Protein binding assays (plasma, microsomal), tissue-specific modeling | Determines drug availability at target sites and potential off-target effects. |
| Metabolism | Liver microsomes, CYP450 enzyme testing, metabolite identification | Assesses metabolic stability and flags toxic or active metabolites. |
| Excretion | Renal/biliary clearance studies, transporter interaction analyses | Predicts elimination pathways and potential accumulation risks. |
Key Methodologies in In Vitro ADME Profiling
Modern ADME profiling leverages a suite of cutting-edge methodologies to generate high-quality data efficiently:
- High-Throughput Screening (HTS): HTS platforms enable rapid assessment of multiple compounds, prioritizing those with favorable ADME properties. Thermodynamic solubility assays (e.g., shake-flask method) and metabolic stability screens (using liver microsomes or hepatocytes) are standard HTS tools. For example, metabolic stability assays measure the percentage of compound remaining after incubation, with half-lives >1 hour typically indicating suitability for further development.
- Advanced Models: Passive permeability assays like PAMPA and cell-based models (e.g., Caco-2) simulate intestinal absorption, while 3D organ-on-a-chip systems replicate human tissue interactions for more physiologically relevant predictions.
- PAMPA and Caco-2 Assays: As mentioned, these models predict intestinal absorption, with Caco-2 cells offering higher physiological relevance due to their expression of transporters and enzymes.
- 3D Organ-on-a-Chip Systems: These microphysiological models, such as liver-on-a-chip, recapitulate tissue architecture and cellular interactions, providing more human-relevant data than 2D cultures. For instance, liver-on-a-chip can simulate bile canalicular excretion and drug-induced hepatotoxicity.
- Automation and Integration: Liquid handling robots and high-performance liquid chromatography-mass spectrometry (LC-MS/MS) have revolutionized ADME profiling. Automated workflows reduce human error and increase throughput, while LC-MS/MS enables sensitive quantification of compounds and metabolites, with detection limits often below 1 nM.
These methodologies not only enhance predictive accuracy but also align with the growing demand for scalable, cost-effective solutions in early drug discovery.
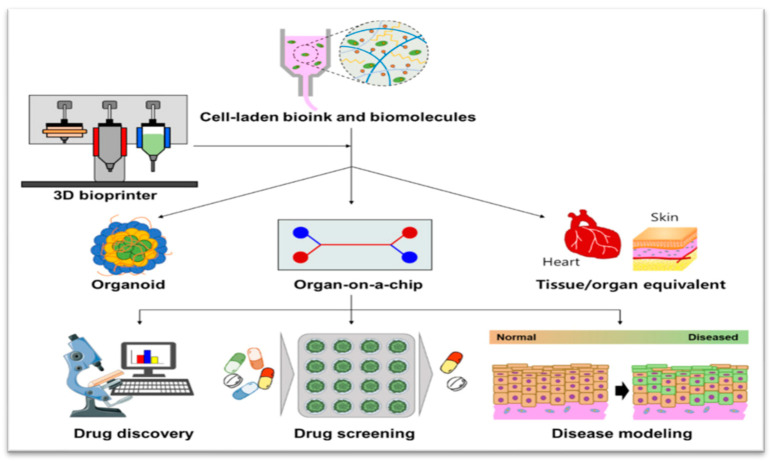 Fig.1 3D bioprinting for advanced pharmaceutical models. 1,2
Fig.1 3D bioprinting for advanced pharmaceutical models. 1,2
Impact of ADME Data Across Drug Development Phases
ADME data influences every stage of drug development:
- Preclinical Research: Early screening eliminates candidates with poor PK properties, saving resources. Structural modifications guided by ADME insights improve bioavailability and reduce toxicity risks.
- Clinical Transition: Data on metabolic pathways and drug-drug interactions inform dose selection and safety monitoring, minimizing adverse events in trials.
- Regulatory Submissions: Comprehensive ADME datasets validate a drug’s safety profile, supporting regulatory approvals and fostering stakeholder confidence.
By addressing PK challenges proactively, ADME profiling mitigates risks and enhances the likelihood of clinical success.
Trends in In Vitro ADME Technology
The field is evolving rapidly, driven by technological and regulatory advancements:
- AI/ML Integration: Machine learning models predict ADME-Tox profiles by analyzing vast chemical datasets, enabling cross-species extrapolation and reducing reliance on animal testing.
- Biomimetic Systems: Organ-on-a-chip platforms and induced pluripotent stem cell (iPSC)-derived models replicate human physiology, offering species-specific insights.
- Regulatory Innovations: Harmonization of testing guidelines, such as FDA-endorsed assays, ensures consistency and reliability in data generation.
These trends underscore a shift toward human-centric, data-driven approaches in ADME science.
If you want to learn more about the transgenic mice, please refer to:
- Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
- In Vitro ADME Screening: Accelerating Drug Development
- In Vitro ADME Assays: Principles, Applications, and Protocols
- Differences and Connections Between ADME and DMPK in Drug Research
- In Vitro ADME and In Vivo PK Studies: A Holistic Framework for Drug Evaluation
- ADME in Toxicology: Ensuring Drug Safety and Efficacy
Comparative Analysis of Leading Service Providers
Creative Biolabs offers comprehensive in vitro ADME (Absorption, Distribution, Metabolism, and Excretion) profiling services to accelerate drug discovery and development. Our advanced platform integrates cutting-edge technologies and expertise to evaluate the pharmacokinetic properties of drug candidates, ensuring optimal efficacy, safety, and bioavailability.
Key Services Include:
- Absorption Studies: High-throughput assays to assess membrane permeability (e.g., Caco-2, PAMPA models) and transporter interactions (e.g., P-gp, BCRP).
- Distribution Profiling: Plasma protein binding (PPB), tissue distribution analysis, and blood-brain barrier (BBB) penetration evaluation.
- Metabolism Assessment: Metabolic stability screening using liver microsomes, hepatocytes, or recombinant CYP enzymes; identification of metabolites via LC-MS/MS; and CYP inhibition/induction studies.
- Excretion Prediction: Renal/biliary clearance mechanisms and transporter-mediated excretion studies.
Conclusion: Future of In Vitro ADME Profiling
The future of in vitro ADME profiling lies in its ability to evolve alongside emerging scientific and regulatory demands. AI-driven optimization tools will refine predictive models, enabling personalized medicine through patient-specific PK simulations. Meanwhile, advancements in biomimetic technologies will reduce reliance on traditional in vivo studies, aligning with global efforts to promote ethical research practices. By continuing to integrate innovation with practicality, ADME profiling will remain indispensable in reducing attrition rates, accelerating timelines, and delivering safer, more effective therapies to patients worldwide.
As drug discovery grows increasingly complex, the strategic value of early ADME insights cannot be overstated. These services not only illuminate the path to clinical success but also embody the collaborative spirit of scientific progress—bridging disciplines, technologies, and visions for a healthier future.
References
- Mihaylova, Anna, et al. "(3D) Bioprinting—Next Dimension of the Pharmaceutical Sector." Pharmaceuticals 17.6 (2024): 797. https://doi.org/10.3390/ph17060797
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
