DMPK & Antibody Drugs
Before the emergence of biomacromolecule drugs, especially antibody drugs, DMPK research was mainly centered around small molecule drugs. However, the emergence of biomacromolecule drugs has brought unprecedented challenges and opportunities to the DMPK field. When drugs are upgraded from relatively small chemical molecules (such as the well-known aspirin) to large macromolecular antibodies (such as PD-1 inhibitors in tumor treatment), the research methods and concepts of DMPK must be "changed". After all, it is completely different to make a "giant protein" circulate in the body in an orderly manner and to let small molecules easily "drill" through the intercellular space.
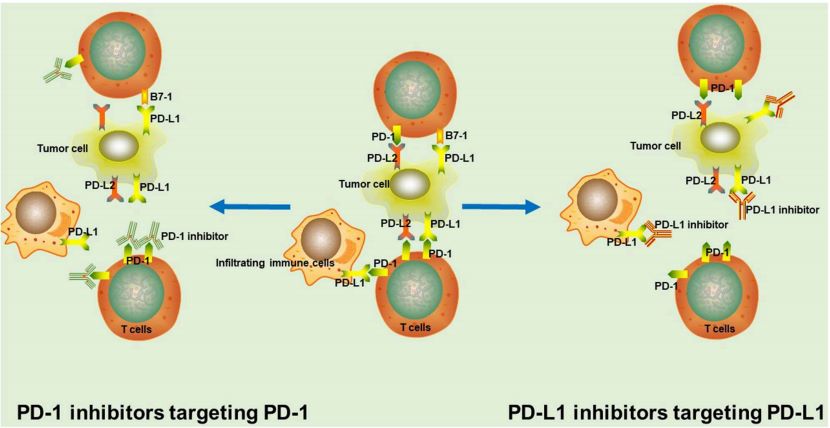 Fig. 1 The rationale of PD-1/PD-L1 inhibitors. In tumor tissue.1,4
Fig. 1 The rationale of PD-1/PD-L1 inhibitors. In tumor tissue.1,4
DMPK properties of antibody drugs: three major differences from small molecules
1.The metabolic pathway of antibody drugs
In the process of drug metabolism, traditional small molecule drugs and antibody drugs take completely different paths. Most small molecule drugs have to rely on the liver to "deal with the aftermath". The cytochrome P450 enzyme system in liver cells is like a chemical plant that runs day and night. Through complex processes such as oxidation, reduction, and hydrolysis, it transforms drugs into substances that can be easily excreted from the body. Antibody drugs are different. As proteins, their metabolic processes are spread throughout the body. Take adalimumab, which is commonly used in clinical practice, for example. Its decomposition in the body is the result of "teamwork" of systemic proteases, and the liver is only responsible for about 20% of the work. This has led to the classic DMPK test method that has been used for many years to evaluate the metabolic capacity of the liver, which is completely useless in front of antibody drugs.
Fortunately, researchers have found a new way - radioactive isotope labeling technology. After "labeling" antibody drugs, following these radioactive signals, they can accurately grasp their degradation rate in various organs and tissues, and accumulate key evidence for clarifying the metabolic pathway of antibody drugs.
2.The distribution mode of antibody drugs
After small molecule drugs enter the blood circulation, they are gradually distributed to various tissues and organs throughout the body through simple diffusion. The distribution of antibody drugs depends on their specific binding to receptors on the surface of target cells. In the field of anti-cancer, in order to target cancer cells and exert drug effects, many antibodies have receptors that are specifically targeted at overexpressed on the surface of tumor cells. For example, anti-HER2 antibodies have extremely high affinity for HER2 receptors on the surface of breast cancer cells. Once bound, antibodies will "aggregate" in tumor tissues in large quantities, resulting in a relatively low concentration of free antibodies in the blood. This unique distribution method makes it impossible for traditional association models based on "blood drug concentration-effect" to accurately predict the efficacy of antibody drugs. Tissue distribution imaging technology just makes up for this shortcoming, and positron emission tomography (PET) is one of the best. PET scanning can clearly and intuitively present the accumulation of antibodies in tumors and other tissues by labeling antibodies with radioactive tracers, providing strong support for evaluating the distribution and efficacy of antibody drugs.
3.The excretion pathway of antibody drugs
Small molecule drugs, due to their small molecular weight, can successfully pass through the glomerular filtration barrier after completing metabolism in the body. These drugs either remain in their original form or are converted into metabolites and excreted from the body through urine. Antibody drugs, however, face a completely different excretion dilemma. The molecular weight of this type of drug is as high as 150 kDa, far exceeding the size threshold of substances allowed to be filtered by the glomerulus, making this conventional excretion channel of the kidney "blocked". Therefore, in the body, antibody drugs mainly enter cells through endocytosis, and then gradually decompose in organelles such as lysosomes to complete the metabolic process. However, there are also some special mechanisms in the excretion process. Taking newborns as an example, the neonatal Fc receptor (FcRn) in their bodies plays a unique role. It can "recycle" antibodies transmitted from the mother through the placenta, prolong the half-life of antibodies in the newborn, and enhance the immunity of the newborn. This special mechanism also provides inspiration for the design of long-acting antibody drugs. Researchers have tried to modify antibody molecules so that they can better utilize the recycling mechanism of FcRn, thereby prolonging the duration of drug action in the body.
Service you may interested in
The "special weapon" for DMPK research of antibody drugs
1. Ligand Binding Assay (LBA): A macromolecule-specific technology that replaces LC-MS
In the field of small molecule drug analysis, liquid chromatography-mass spectrometry (LC-MS) technology has long occupied the core position of determining drug concentration with its excellent high sensitivity and high resolution, and can be called the "gold standard" for small molecule drug analysis. However, once the research focus shifts to macromolecular drugs such as antibodies, the shortcomings of LC-MS technology are exposed. The structure of antibody drugs is particularly complex, the three-dimensional shape is ever-changing, and the physical and chemical properties are also very special. When using LC-MS for detection, these characteristics are like checkpoints: either the peaks in the detection spectrum become wide and scattered, or the efficiency of the ionization process is greatly reduced. In this way, it becomes a big problem to accurately measure the content of antibody drugs.
In such a dilemma, ligand binding analysis (LBA) stands out like a dazzling new star and becomes a powerful tool for DMPK research of antibody drugs. The principle of LBA is cleverly based on the immunological reaction of antigen-antibody specific binding. Researchers carefully prepare highly specific antibodies as "bait". These "bait" can be like precise "molecular catchers". According to the highly specific recognition mechanism between antigen and antibody, they can accurately "capture" drug antibodies in the sample. Subsequently, the combined complex is carefully detected by advanced detection techniques such as enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence. Taking ELISA as an example, it uses enzyme-labeled antibodies to specifically bind to antigen-antibody complexes, and uses enzyme-catalyzed substrates to develop colors. According to the corresponding relationship between the color depth and the antibody drug concentration in the sample, the concentration of antibody drugs in the sample can be accurately determined.
Compared with LC-MS, LBA has unparalleled compatibility and specificity for macromolecular drugs. Taking the detection of PD-1 antibody concentration in the blood of cancer patients as an example, the cancer patient's own immune system will produce PD-1 antibodies, and LBA has a highly specific antibody design that can keenly distinguish drug antibodies from the PD-1 antibodies produced by the patient himself, which can effectively avoid cross-interference and ensure the accuracy and reliability of the test results. Provide solid data support for the formulation of clinical treatment plans.
2. Cell binding experiment: Evaluate the affinity of antibodies to target receptors
The affinity between antibodies and target receptors is the core key factor that determines the efficacy and in vivo distribution of antibody drugs. Therefore, cell binding experiments play a pivotal role in antibody DMPK research and can be called a "must-test question" in the research process. In the cell binding experiment, researchers place cells expressing target receptors and antibodies with different concentration gradients in a suitable incubation environment to allow the antibodies to fully contact and interact with the target receptors on the cell surface.
In order to accurately determine the degree of binding between antibodies and cell surface receptors, researchers have used a variety of advanced detection methods. For example, fluorescent labeling technology covalently binds fluorescent substances to antibodies. When antibodies bind to target receptors, with the help of equipment such as fluorescence microscopes or flow cytometers, the intensity of fluorescent signals can be intuitively observed and accurately measured, thereby reflecting the amount of binding between antibodies and receptors; flow cytometry can quickly and high-throughput analyze a large number of cells, and obtain multiple parameter information of cells at the same time, including the degree of binding between antibodies and receptors, the activity state of cells, etc.
As an important quantitative indicator for measuring antibody affinity, the binding dissociation constant (KD) plays a key role in evaluating the performance of antibody drugs. By definition, the smaller the KD value, the stronger the binding ability of the antibody to the receptor. Therefore, in the early stages of DMPK research, researchers will conduct cell binding experiments on a large number of candidate antibodies, and by accurately measuring the KD value, give priority to molecules with high affinity to enter the subsequent research and development process, thereby improving research and development efficiency and reducing research and development costs.
3. Transgenic animal models: solving the problem of species differences
In the long journey of drug development, animal models have always been an indispensable and important tool. They provide valuable preliminary data for the study of the mechanism of action and safety evaluation of drugs in vivo. However, for antibody drugs, species differences are like a huge obstacle on the road to research and development, bringing great challenges. The FcRn (neonatal Fc receptor) in ordinary mice, which are commonly used in drug development, is quite different from that in humans, both in structure and function. This also causes the same antibody drug to disappear very quickly in the mouse body. Because of the huge difference in metabolic rates, it is difficult for scientists to truly restore the metabolic process and effects of antibody drugs in the human body through mouse experiments. For example, a drug that can last for several days in the human body may be metabolized in a few hours in mice. The predictions based on this data are often very different from the actual situation. This not only makes every step of drug development like groping in the fog, repeatedly adjusting the experimental plan, but also greatly lengthens the research and development cycle, making it even more difficult to successfully develop an effective antibody drug. To solve this problem, the researchers successfully constructed personalized FcRn mice using gene editing technology. The researchers replaced the mouse's own FcRn gene with the human FcRn gene, thereby "transforming and upgrading" the mouse's immune system, causing a fundamental change in the antibody metabolic environment in the mouse, which is closer to the human physiological state. In this model, the half-life of the antibody is closer to the half-life of humans, and can more realistically simulate the pharmacokinetic process of antibody drugs in the human body, including drug absorption, distribution, metabolism, excretion, etc. By conducting preclinical studies on the "humanized FcRn mouse" model, researchers can more accurately evaluate the efficacy and safety of antibody drugs, provide a reliable basis for the design and optimization of subsequent clinical trial programs, greatly improve the success rate of antibody drug research and development, and accelerate the pace of innovative antibody drugs from the laboratory to clinical application. In the DMPK research of antibody drugs, the three "special weapons" of ligand binding assay (LBA), cell binding experiment and transgenic animal model each play a unique and irreplaceable important role. LBA solves the problem of accurate determination of antibody drug concentration by virtue of its high specificity and compatibility with macromolecular drugs; cell binding experiment provides a key basis for screening highly effective antibody drugs by accurately evaluating the affinity between antibodies and target receptors; transgenic animal models effectively overcome the obstacles of species differences and build a reliable bridge from preclinical research to clinical trials. These technical means cooperate with each other and complement each other, and together provide solid technical support for the research and development of antibody drugs, driving the field of antibody drug research and development forward, and bringing new hope for conquering major diseases and improving human health.
Optimize Your Candidate with Confidential DMPK Analysis
At Creative Biolabs, we offer comprehensive DMPK analysis for your candidate compounds. We know every research project is unique, so we'll work with you to select the most suitable strategy based on your specific study characteristics. Rest assured, all your data will be kept strictly confidential.
Ready to discuss your DMPK needs and find the perfect solution? Contact us today!
If you want to learn more about the DMPK, please refer to:
References
- Chen, Yu, et al. "Looking for the optimal PD-1/PD-L1 inhibitor in cancer treatment: a comparison in basic structure, function, and clinical practice." Frontiers in immunology 11 (2020): 1088. https://doi.org/10.3389/fimmu.2020.01088
- Wan, Hong. "An overall comparison of small molecules and large biologics in ADME testing." Admet and Dmpk 4.1 (2016): 1-22. https://doi.org/10.5599/admet.4.1.276
- Tsuchikama, K., and Z. An. "Antibody-drug conjugates: recent advances in conjugation and linker chemistries." Protein Cell 9: 33–46." 2018, https://doi.org/10.1007/s13238-016-0323-0
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
