Alloxan induced Diabetes Models: Protocols, Mechanisms & Applications
Introduction to Alloxan-Induced Diabetes Models
Alloxan (CAS 50-71-5), a pyrimidine derivative with a molecular weight of 142.07 g/mol, was first identified in 1943 and quickly became a pivotal tool in diabetes research. Its discovery revolutionized the field by enabling the induction of experimental diabetes in animals, providing a tractable model to study disease pathophysiology and test therapeutic strategies.
Alloxan induces diabetes through selective β-cell toxicity, primarily via the generation of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide (H₂O₂), and hydroxyl radicals. This oxidative stress disrupts β-cell function and viability. Additionally, alloxan inhibits glucokinase, impairing glucose sensing and leading to insulin deficiency. While the model primarily mimics acute β-cell destruction, its pathophysiology shares similarities with the insulin deficiency seen in human type 1 diabetes, though it lacks the autoimmune component of the human disease.
Step-by-Step Protocols for Model Development
Alloxan Diabetic Mouse Model
Animal Selection
Male Kunming mice (6–8 weeks old, 30±5 g) are preferred for their consistency in responding to alloxan.
Dosage & Administration
Administer 75–100 mg/kg of alloxan via tail vein injection as a 1–3% saline solution, freshly prepared to maintain stability.
Fast mice for 24 hours prior to injection to enhance β-cell sensitivity to alloxan.
Monitoring
Diabetes is confirmed when blood glucose exceeds 200 mg/dL (11.1 mmol/L) 72 hours post-injection.
Long-term stability requires sustained hyperglycemia (>16.7 mmol/L) over a 2-week observation period.
Alloxan Diabetic Rat Model
Optimal Dosing
Dosing varies by strain, with Sprague-Dawley (SD) rats typically receiving 60 mg/kg intravenously. Ranges from 60–200 mg/kg have been reported, but strain-dependent toxicity must be considered.
Critical Steps
Post-injection glucose supplementation is essential to prevent fatal hypoglycemia. Administer 25% glucose water 6 hours after alloxan injection to improve survival rates.
Validation
Urine glucose ≥++ and blood glucose ≥6.1 mmol/L at 15 days confirm successful model establishment.
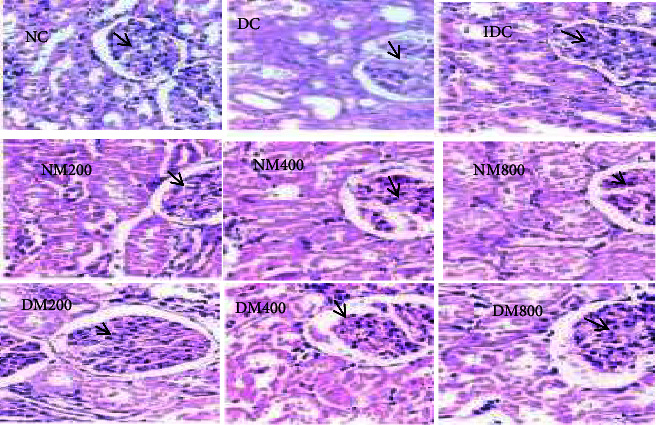 Fig.1 Renal effects of MO in diabetic animals.1,2
Fig.1 Renal effects of MO in diabetic animals.1,2
Key Parameters for Type 1 Diabetes Induction
Dose Optimization
Species-specific dosage thresholds are critical: mice typically require 30–40 mg/kg intravenously, while rats need 40–200 mg/kg. High doses pose risks of ketosis and hepatic/renal toxicity, necessitating careful titration to balance model efficacy and animal welfare.
Monitoring Biomarkers
Primary Metrics
Fasting blood glucose (FPG), glycated hemoglobin (HbA1c), and insulin/C-peptide levels directly reflect glycemic control and β-cell function.
Urine glucose strips offer a rapid, non-invasive method for initial screening.
Secondary Markers
Oxidative stress markers, such as malondialdehyde (MDA) and glutathione (GSH), quantify cellular damage. Alloxan-induced models show significantly elevated MDA (118.9 nmol/L vs. 70.35 nmol/L in controls) and reduced GSH (1.01±0.12 mmol/L vs. 2.44±0.02 mmol/L), reflecting heightened oxidative stress.
β-cell histopathology and nitric oxide (NO) levels further characterize disease progression.
Service you may interested in
Alloxan vs. STZ: A Comparative Analysis
Alloxan and streptozotocin (STZ) are the two most widely used chemical diabetogens, but they differ profoundly in mechanism and application. Alloxan acts via ROS-mediated β-cell necrosis, while STZ induces DNA alkylation and mitochondrial damage. Alloxan has a half-life of 1.5 minutes, significantly shorter than STZ’s 15 minutes, contributing to its transient hyperglycemia (lasting <1 month vs. up to 3 months for STZ). Cost-wise, alloxan is far more economical ($6.75/g vs. $511/g for STZ), making it suitable for large-scale studies. However, it carries a higher mortality rate due to ketosis risk, whereas STZ is better tolerated. Alloxan is ideal for acute studies and antioxidant research, while STZ suits chronic models and genetic studies (see Table 1).
Table 1: Key Differences Between Alloxan and STZ Models
| Parameter | Alloxan | STZ |
|---|---|---|
| Mechanism | ROS-mediated β-cell necrosis | DNA alkylation + mitochondrial damage |
| Half-Life | 1.5 minutes | 15 minutes |
| Cost | $6.75/g | $511/g |
| Hyperglycemia Duration | <1 month | Up to 3 months |
| Mortality Rate | High (ketosis risk) | Lower |
| Applications | Acute studies, antioxidant research | Chronic models, genetic studies |
Research Applications & Case Studies
Drug Testing
Alloxan models have been pivotal in evaluating antidiabetic agents. For example, an extract from diapensia himalaea reduced FPG by 21.64% in rats over a 28-day trial, demonstrating its hypoglycemic potential. Similarly, ganoderma species exhibit anti-hyperglycemic effects by scavenging ROS, as shown by reduced MDA levels and improved glucose tolerance.
Pathological Mechanism Studies
Oxidative Stress
Alloxan-induced MDA elevation (118.9 nmol/L vs. 70.35 nmol/L in controls) underscores the role of oxidative damage in β-cell dysfunction.
β-Cell Regeneration
Unlike STZ models, alloxan models show failed α-to-β cell conversion, highlighting limitations in studying β-cell regeneration.
Protective Agents
Reduced water (RW) has been shown to prevent alloxan-induced ATP depletion in β-cells, offering insights into cytoprotective strategies.
Advantages, Limitations, and Best Practices
Alloxan models offer distinct advantages: they are cost-effective for large-scale studies and enable rapid diabetes induction (18–72 hours). However, they require careful management: high mortality rates occur without glucose support, and hyperglycemia is transient in rodents. To optimize models, use male animals, fast for 12 hours pre-injection, and avoid guinea pigs, which exhibit innate alloxan resistance.
If you want to learn more about the diabetic animal models, please refer to:
Future Directions and Conclusion
Future research may focus on combining alloxan with STZ to create stable, low-toxicity models, integrating omics technologies (e.g., single-cell RNA-seq) to dissect β-cell death pathways, and developing humanized mouse models for translational studies. Despite limitations, alloxan remains irreplaceable in type 1 diabetes research, providing a rapid, economical platform to advance mechanistic understanding and therapeutic discovery. Standardized protocols are essential to improve reproducibility and accelerate scientific progress in this field.
References
- Amina, Egbujo Ejike, et al. "Hypoglycemic Assessment of Aqueous Leaf Extract of Moringa oleifera on Diabetic Wistar Rats." Biochemistry Research International 2024.1 (2024): 9779021. https://doi.org/10.1155/2024/9779021
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
