Humanized Mice Models: From COVID-19 to Cancer - Key Applications
Humanized mouse models have emerged as indispensable tools in biomedical research, enabling scientists to simulate human biological processes and diseases within a murine framework. These models overcome the species-specific barriers that have long hindered translational medicine, providing a critical bridge between basic research and clinical application. From unraveling the mechanisms of viral infections like COVID-19 to advancing cancer immunotherapy, humanized mice have revolutionized how we study and tackle complex human diseases.
Introduction: Defining Humanized Mouse Models and Their Scientific Value
Definitions and Classifications
Humanized mouse models are created by integrating human genetic, cellular, or tissue components into immunodeficient mice, allowing for the recapitulation of human physiological responses. They can be categorized into three primary types:
Gene-Edited Humanized Models
Using technologies like CRISPR-Cas9, murine genes are replaced with their human orthologs. For instance, inserting the human ACE2 gene (angiotensin-converting enzyme 2) enables mice to express the receptor critical for SARS-CoV-2 entry, while humanizing immune checkpoint genes (e.g., PD-1, CTLA-4) facilitates the study of cancer immunotherapy.
Immune System Humanized Models
These models reconstitute human immune components:
- Hu-PBMC Models: Human peripheral blood mononuclear cells (PBMCs) are transplanted to rapidly rebuild T cell responses, suitable for short-term immune studies.
- Hu-HSC Models: Human hematopoietic stem cells (HSCs) are introduced to establish a long-term, functional immune system, supporting innate immunity research.
- Hu-BLT Models: A more complex approach involving transplantation of human bone marrow, liver, and thymus tissues to mimic adaptive immunity, though with higher risks of graft-versus-host disease (GVHD).
Tissue and Tumor Humanized Models
- Patient-Derived Xenografts (PDX): Tumor tissues from patients are implanted into mice, preserving the native tumor microenvironment.
- Cell Line-Derived Xenografts (CDX): Established cancer cell lines are used for standardized drug efficacy testing.
Core Value in Translational Research
By emulating human biological systems, humanized mice address a critical gap in preclinical research. They allow scientists to:
- Study disease mechanisms that are otherwise unfeasible in humans,
- Test the safety and efficacy of novel therapeutics before clinical trials,
- Bridge the translational gap between basic science and clinical application.
Service you may interested in
Key Applications in COVID-19 Research
Modeling Infection Mechanisms and Symptoms
ACE2 Humanized Models
Expressing the human ACE2 receptor, these models have been pivotal in understanding SARS-CoV-2 pathogenesis:
- Researchers developed models that recapitulate mild COVID-19 symptoms, including respiratory inflammation and viral replication patterns.
- Optimized models with stable hACE2 expression have been used to monitor long-term post-infection sequelae, shedding light on "long COVID" mechanisms.
Immune Response Studies
Models with humanized immune systems have revealed how genetic and immunological background differences influence disease severity. For example, they helped identify immune pathways contributing to cytokine storms and viral clearance, guiding the development of anti-inflammatory therapies.
Vaccine and Drug Evaluation
Vaccine Immunogenicity Testing
Humanized mice expressing human leukocyte antigens (HLAs) have been instrumental in evaluating vaccine-induced T cell responses. These models confirm whether vaccines elicit robust interferon-γ (IFN-γ) secretion and antigen-specific immunity, critical for predicting clinical efficacy.
Therapeutic Antibody Development
ACE2 humanized mice serve as gold-standard platforms for assessing neutralizing antibodies. They enable researchers to measure how effectively antibodies block viral entry, supporting the rapid development of monoclonal antibody therapies during the pandemic.
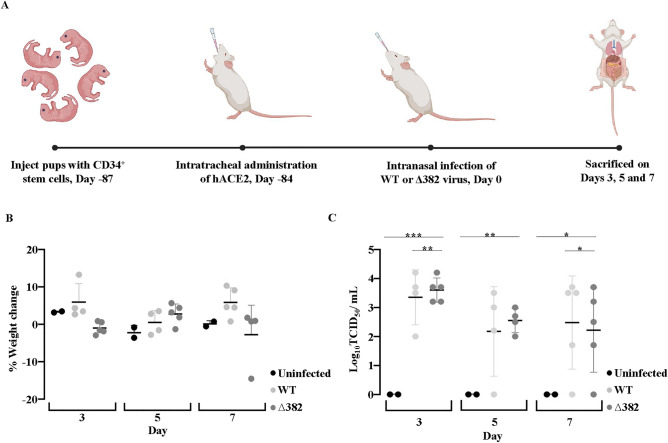 Fig.1 hACE2-transduced humanized mice support SARS-CoV-2 infection.1,2
Fig.1 hACE2-transduced humanized mice support SARS-CoV-2 infection.1,2
Core Applications in Cancer Research
Recreating the Tumor Microenvironment
Dual-Humanized PDX Models
The process involves:
- Transplanting human HSCs into immunodeficient mice (e.g., NSG mice) to reconstitute a human immune system,
- Implanting patient-derived tumor tissues to form a "humanized" tumor microenvironment.
This approach preserves interactions between human immune cells and tumor cells, allowing researchers to study tumor-immune crosstalk and immune evasion mechanisms.
Immunotherapy Evaluation
Humanized models are essential for preclinical testing of cancer immunotherapies:
- They validate the efficacy of CAR-T cell therapies and bispecific antibodies, which target human-specific antigens.
- They help optimize treatment protocols by modeling how human immune cells respond to therapeutic interventions.
Targeted Therapy and Drug Resistance Research
Immune Checkpoint Humanized Models
Mice with humanized PD-1/PD-L1 pathways have been crucial for developing immune checkpoint inhibitors. These models also help identify resistance mechanisms, guiding the design of combination therapies.
Metastasis Research
PDX models combined with humanized immune systems shed light on how tumors metastasize and evade immune surveillance. This knowledge informs strategies to prevent metastasis and enhance immune-mediated tumor clearance.
Advantages in Antibody Production and Vaccine Development
Fully Human Antibody Discovery
Technological Comparisons
| Platform | Advantages | Limitations |
|---|---|---|
| Fully Humanized Mouse Technology | High-affinity antibodies, no need for humanization, low immunogenicity | Target bias, challenges in automation |
| Phage Display Technology | Rapid screening across multiple targets | Lower affinity, potential patent disputes |
Fully humanized mouse platforms have dominated the market, contributing to ~70% of approved fully human monoclonal antibodies. Their ability to generate antibodies that mimic human immune responses reduces the risk of adverse reactions in patients.
Vaccine Validation Mechanisms
Immune Response Assessment
- HLA-humanized mice enable the identification of T cell epitopes critical for vaccine-induced immunity.
- Models like NOD/SCID/IL2Rγnull mice support studies on human dendritic cell activation and antibody durability, ensuring vaccines elicit long-lasting protection.
Common Technologies and Challenges Across COVID-19 and Cancer Models
Shared Technical Foundations
Receptor Humanization
Both fields rely on gene editing to humanize key receptors: ACE2 for COVID-19 research and immune checkpoint molecules (e.g., PD-1) for cancer studies.
Immunodeficient Hosts
Strain-specific mice like NSG and NOG serve as universal platforms, providing a blank canvas for human cell and tissue engraftment.
Overlapping Pathological Mechanisms
- ACE2 in Cancer: ACE2 overexpression in lung cancer cells has been linked to increased susceptibility to severe COVID-19, highlighting cross-disease relevance.
- Inflammatory Pathways: Signaling pathways like IL-6/STAT3 play roles in both COVID-19 cytokine storms and tumor progression, presenting opportunities for shared therapeutic strategies.
Challenges and Innovations
Current Limitations
- GVHD remains a hurdle in immune system humanized models,
- Immune cell development in some models may not fully recapitulate human physiology.
Future Directions
- Dual Gene Editing: Combining hACE2 with hTMPRSS2 (a protease) enhances SARS-CoV-2 infection efficiency in models.
- Cytokine Humanization: Introducing human cytokines (e.g., IL-3, GM-CSF) improves immune cell functionality and engraftment.
Conclusion and Future Perspectives
Humanized mouse models have transformed translational research, enabling breakthroughs in both infectious disease and cancer therapy. Their ability to mirror human biology has accelerated the development of life-saving vaccines, antibodies, and immunotherapies.
Looking ahead, the field is moving toward:
- Complex Multiorgan Models: Integrating multiple human tissues or organoids to simulate systemic diseases,
- AI-Driven Phenotyping: Using artificial intelligence to analyze model data and predict clinical outcomes,
- Standardization and Ethics: Establishing global guidelines for model development and experimental practices to ensure reproducibility and ethical conduct.
As technology advances, humanized mice will continue to serve as indispensable tools, bridging the gap between laboratory discoveries and personalized medicine for patients worldwide.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Yong, Kylie Su Mei, et al. "Comparison of infection and human immune responses of two SARS-CoV-2 strains in a humanized hACE2 NIKO mouse model." Scientific Reports 13.1 (2023): 12484. https://doi.org/10.1038/s41598-023-39628-y
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
