DMPK in Clinical Drug Development
The first condition for a drug to be effective is to determine the dosage. When a new anticancer drug shows an anticancer effect at a dose of 10 mg/kg in mice, what should be the safe dose for it to be effective in humans? DMPK plays the role of "cross-species translation" in this process, using a rigorous dose conversion logic to solve the problem. Taking body surface area conversion as an example, the body surface area coefficient of rats is 6, and that of humans is 37. According to the formula, human equivalent dose = rat dose *rat body surface area coefficient /human body surface area coefficient, the rat dose of 10mg/kg is converted to about 1.6mg/kg in humans. But this is only a theoretical "draft answer" - in the first human trial (FIH), 6 to 12 volunteers are like real-world "calculators" to help verify dose safety. DMPK uses data to pave the way for drugs from animal experiments to human applications.
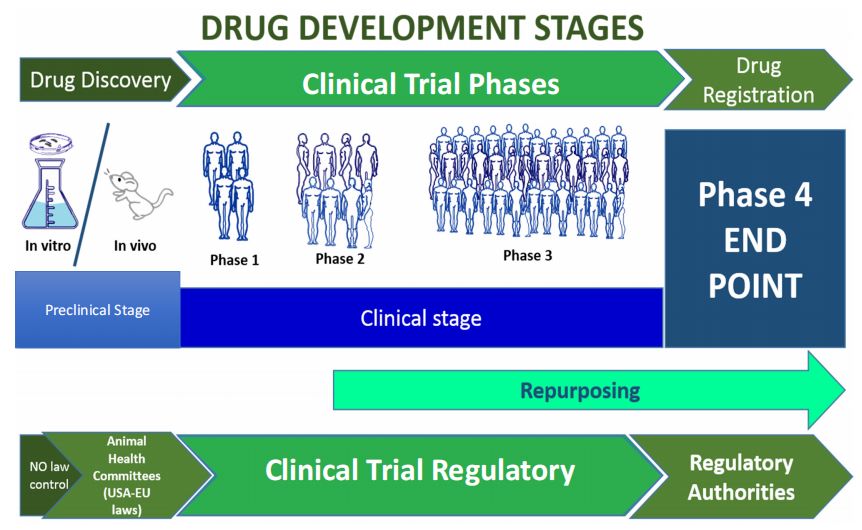 Fig. 1 Drug development and clinical trial stages.1,4
Fig. 1 Drug development and clinical trial stages.1,4
"Safety Sentinels" in Clinical Trials
In the long clinical trials, DMPK is like a tireless "safety sentinel", escorting drug safety by continuously monitoring blood drug concentrations. Let's take cancer treatment as an example. Different patients have huge differences in their ability to metabolize chemotherapy drugs. This is mainly reflected in the fact that patients with CYP2D6 gene mutations metabolize paclitaxel three times slower than ordinary people. This means that if patients are treated with standard doses, blood drug concentrations will "explode" like a runaway train, causing a serious risk of bone marrow suppression. To avoid this risk, we need DMPK to monitor blood drug concentrations in real time and adjust the dose to 50% of the standard dose, so that we can ensure the anti-cancer effect while avoiding fatal side effects. For patients with organ dysfunction, DMPK has a more "customized" value: the creatinine clearance rate of patients with renal failure is like an "excretion rate meter". Doctors calculate the antibiotic dose based on the DMPK principle, just like accurately irrigating water-deficient farmland to prevent drugs from accumulating in the body like stagnant water. In the scenario of multi-drug combination, DMPK becomes the "drug metabolism traffic coordinator" - when statins and clarithromycin are taken together, DMPK finds that clarithromycin will "block" the "highway" (CYP3A4 enzyme) of statin metabolism, causing the statin blood concentration to soar 5 times and increase the risk of muscle toxicity. Based on this discovery, doctors promptly advise patients to stagger the time of taking medicine, successfully clearing the "congestion point" of drug metabolism.
Service you may interested in
The "Gene Decoder" for Personalized Medicine
The diversity of human genes has gradually made the era of "one drug for a thousand people" a thing of the past, and DMPK is the core driving force behind personalized medicine. About 20% of Asians are "slow metabolizers" of CYP2C19. When taking omeprazole, the drug clearance rate is like "snail crawling", and half of the conventional dose can achieve acid suppression effect. DMPK combines genetic testing with blood drug concentration monitoring to draw a unique "drug metabolism map" for each patient, just like navigation software plans personalized routes for different drivers. Medication during pregnancy can better reflect the "customized" wisdom of DMPK: the physiological changes in pregnant women are like "speed gears of drug metabolism". For example, carbamazepine, which is used to treat epilepsy, will be metabolized faster during pregnancy, and only increased dosage can maintain effective concentration; and when the antibiotic penicillin is used during pregnancy, renal excretion will also be accelerated, and the frequency of administration must be adjusted to ensure the bactericidal effect. This precise regulation based on individual differences allows the drug to achieve maximum efficacy while avoiding toxic "minefields".
"Warning beacons" of clinical failure
On the thorny road of drug development, every decision concerns the life and health of countless patients. Clinical failure cases caused by ignoring DMPK (drug metabolism and pharmacodynamics) warnings are like reefs, lurking under the seemingly calm surface of research and development, reminding researchers at all times. Take the development process of a new drug for the treatment of heart failure as an example. In the highly anticipated Phase III clinical trial, it was forced to stop urgently due to the cruel reality that the patient mortality rate was higher than that of the placebo group. Looking back on the entire development process, it is like uncovering the truth of a heartbreaking "accident". In the preclinical animal experiment stage, DMPK research has found that the drug's metabolite M3 has significant cardiotoxicity. However, driven by the urgent pursuit of efficacy, the research and development team chose to ignore this important warning and insisted on advancing the clinical trial with a fluke mentality. Further in-depth research found that the drug concentration of patients in the high-dose group exceeded the safety threshold by 2 times. This seriously exceeded the drug concentration, which amplified the cardiotoxicity of the metabolite M3, which already had hidden dangers, and ultimately led to an increase in patient mortality, resulting in tragedy. This case is like a lighthouse standing on the vast ocean. With its painful lessons, it clearly and strongly illuminates the key value of DMPK in clinical decision-making. DMPK is not just a cold data calculation tool. It is a "red line" to protect patient safety and an insurmountable bottom line in the drug development process. In sailing, navigators will never ignore the warning of the lighthouse, because that may mean the destruction of the ship and the death of people; similarly, drug developers must also use DMPK data as an important basis for clinical advancement, always maintain awe, fully respect the metabolic laws of drugs in the human body, and avoid blindly pursuing efficacy and ignoring potential risks.
In fact, in the drug development industry, similar cases of failure due to ignoring DMPK data are not isolated cases. According to industry statistics, clinical failures caused by drug metabolism and safety issues each year account for as much as 30% - 40% of all failed cases. Behind these data is the waste of countless scientific research resources and the disappointment of patients' expectations for new drugs. Every failed case is constantly sounding the alarm, emphasizing the indispensability of DMPK research in drug development.
DMPK and precision medicine
In the era of precision medicine, DMPK is playing an unprecedented important role and building a complete "drug translation ecosystem". From cross-species dose conversion to individual gene decoding, DMPK has built a bridge for drugs to move from the laboratory to clinical application, making drug research and development more scientific and precise.
In the field of cancer immunotherapy, the value of DMPK has been fully reflected. Taking PD-1 antibody as an example, its half-life is as long as 21 days. If this feature is not reasonably utilized, it may cause the drug to accumulate in the body and increase the risk of immune-related toxicity; if the dosing interval is too short, it may not achieve the best therapeutic effect. Through DMPK, we deeply analyzed the pharmacokinetic parameters of PD-1 antibodies, such as half-life and receptor occupancy, and combined with clinical research data, designed a dosing regimen of every 2 weeks. This regimen not only ensures that the drug can continue to exert its anti-tumor effect and effectively activate the immune system to fight cancer cells, but also reduces the incidence of immune-related toxicity and significantly improves patients' treatment tolerance and quality of life. In actual clinical applications, the tumor remission rate of patients using this dosing regimen is 15% - 20% higher than that of traditional dosing regimens, and the incidence of severe immune-related adverse reactions is reduced by about 10%.
DMPK also played an indispensable role in the race against time for the development of the new crown mRNA vaccine. mRNA is extremely fragile and easily degraded by nucleases in the human body. How to safely and effectively deliver it to immune organs is a key problem in vaccine development. From the perspective of drug delivery systems, DMPK conducted in-depth research on the characteristics of lipid nanoparticles, including the effects of their particle size, surface charge, and composition on mRNA stability and delivery efficiency. Through a large number of experiments and data analysis, the optimization design of lipid nanoparticles was guided, and the problem of RNA being easily degraded was successfully solved, allowing the vaccine to accurately reach immune organs, such as lymph nodes, to stimulate the body to produce an effective immune response. The new crown mRNA vaccine finally developed has shown an effectiveness of up to 95% in clinical trials, providing a powerful weapon for the global fight against the new crown epidemic.
These breakthrough results fully demonstrate the dual value of DMPK: it is not only the "gatekeeper" of drug safety, strictly controlling the metabolic process of drugs in the body to ensure the safety and effectiveness of drugs; it is also the "catalyst" of innovative therapies, promoting the continuous advancement of drug research and development technology and accelerating the birth of innovative therapies. With the rapid development of artificial intelligence (AI) and organ chip technology, the future of DMPK is full of infinite possibilities. AI's powerful data analysis and prediction capabilities can quickly process massive amounts of drug metabolism data and establish more accurate pharmacokinetic models; organ chip technology can simulate the physiological environment and function of human organs and more realistically reflect the metabolic process of drugs in the body. Combining the two, DMPK will be able to more accurately "translate" the metabolic profile of drugs in different individuals, fully consider factors such as genetic differences and physiological state differences between individuals, so that each new drug can find the most suitable "language mode" in the human body, truly realize personalized medicine, and bring patients safer and more effective treatment options.
DMPK - the "human translator" of drugs
In the journey of clinical drug development, DMPK is like a senior translator who is proficient in "animal language" and "human language", allowing chemical molecules in the laboratory to "talk" with human physiology. From 10mg/kg in rat experiments to 1.6mg/kg in humans, from standard doses to personalized plans, DMPK uses data and wisdom to build a bridge from the laboratory to the bedside. In the next article, we will focus on the "golden partner" of DMPK and PKPD, analyzing how they work together to decipher the ultimate code of "drug concentration-efficacy relationship" and inject more precise scientific power into new drug research and development.
If you want to learn more about the DMPK, please refer to:
What is DMPK?
DMPK vs PKPD: Collaborative Mechanisms in Drug Development
References
- Meringolo, Maria et al. "Leaflet: Operative Steps for Interventional Studies in Neuroscience." Neurology international vol. 17,1 1. 24 Dec. 2024, https://doi.org/10.3390/neurolint17010001
- Andrade, E L et al. "Non-clinical studies in the process of new drug development - Part II: Good laboratory practice, metabolism, pharmacokinetics, safety and dose translation to clinical studies." Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas vol. 49,12 e5646. 12 Dec. 2016, https://doi.org/10.1590/1414-431X20165646
- Hirabayashi, Hideki. "What is the definitive role of DMPK research for successful drug discovery and development? Until now and in the future." Drug metabolism and pharmacokinetics vol. 29,5 (2014): 357-9. https://doi.org/10.2133/dmpk.dmpk-14-pf-905
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
