Reviews of Diet induced Type 2 Diabetes in C57BL/6J Mice
Introduction to Type 2 Diabetes Mouse Models
Animal models have been indispensable in unraveling the pathophysiology of type 2 diabetes mellitus (T2DM) and evaluating therapeutic strategies. Among these, mouse models stand out for their genetic tractability and metabolic similarity to humans. Two primary categories dominate T2DM research: genetic models, such as db/db mice, which carry inherent mutations, and diet-induced models, which recapitulate disease progression through environmental factors. The C57BL/6J mouse strain has emerged as a gold standard in diet-induced T2DM research, celebrated for its genetic stability, robust metabolic response to dietary interventions, and translational relevance to human disease.
The choice of C57BL/6J mice is rooted in their well-characterized genome and consistent physiological responses. Unlike genetically modified models that may exhibit accelerated disease phenotypes, diet-induced C57BL/6J mice develop T2DM progressively, mirroring the slow onset of insulin resistance and β-cell dysfunction seen in humans. This feature makes them particularly valuable for studying the early stages of metabolic dysregulation and obesity-linked complications.
Development of the Diet-Induced C57BL/6J Mouse Model
Protocol Optimization
The cornerstone of inducing T2DM in C57BL/6J mice is a high-fat diet (HFD) formulated to disrupt energy homeostasis. A typical HFD consists of approximately 60% fat by calorie, with key components including casein (a protein source), lard (a saturated fat), and sucrose (a simple carbohydrate). This composition mimics the Western-style diet associated with rising T2DM prevalence globally. The dietary intervention is usually initiated in young adult mice (8–12 weeks old), with the duration varying from 8 to 20 weeks to achieve distinct stages of disease, from obesity and insulin resistance to overt hyperglycemia.
For researchers seeking to accelerate β-cell dysfunction, a hybrid protocol incorporating low-dose streptozotocin (STZ) has proven effective. STZ, a chemical toxin, selectively damages pancreatic β-cells, and when administered at doses around 50 mg/kg, it complements HFD-induced insulin resistance to mimic advanced T2DM with reduced latency. This approach is particularly useful for studies requiring rapid model establishment while maintaining physiological relevance.
Critical Parameters for Success
Monitoring key metrics is essential to validate model success. Weight gain serves as an initial indicator, with a target rate of 1.5–2 g per week reflecting appropriate dietary response. Metabolic characterization must include fasting glucose levels exceeding 200 mg/dL, abnormal glucose tolerance (assessed via oral glucose tolerance test, OGTT), and insulin sensitivity (evaluated by insulin tolerance test, ITT). Lipid profiles, including triglycerides (TG) and total cholesterol (TC), often exhibit dysregulation, mirroring human dyslipidemia.
Pathological validation is equally critical. Histological analysis typically reveals hepatic steatosis (fat accumulation in the liver), pancreatic islet vacuolization (indicative of β-cell stress), and vascular dysfunction, all hallmarks of T2DM complications. These findings ensure that the model recapitulates not only glycemic abnormalities but also the broader metabolic syndrome.
Comparative Analysis: C57BL/6J vs. db/db Mice
Understanding the strengths and limitations of C57BL/6J-HFD mice requires comparison with their genetic counterparts, db/db mice. The db/db strain carries a mutation in the leptin receptor gene, leading to morbid obesity, rapid-onset hyperglycemia, and severe β-cell failure. In contrast, C57BL/6J-HFD mice develop disease gradually, driven by dietary excess rather than genetic defect.
Disease Progression Dynamics
Db/db mice exhibit hyperglycemia within weeks of weaning, accompanied by profound insulin deficiency and early infertility, making them unsuitable for long-term complication studies. Their aggressive phenotype is ideal for acute drug efficacy testing, particularly for compounds targeting insulin secretion. Conversely, C57BL/6J-HFD mice spend months in a pre-diabetic state of insulin resistance, providing a window to study the underlying mechanisms of obesity-induced metabolic dysfunction. This gradual progression aligns closely with the human trajectory from prediabetes to T2DM, making it invaluable for mechanistic research.
Research Applications
The db/db model shines in late-stage drug testing, where its severe phenotype quickly reveals therapeutic effects on glycemic control. For example, it has been used to evaluate oral hypoglycemic agents, though specific drug names are best avoided to prevent potential infringement. In contrast, the C57BL/6J-HFD model is better suited for investigating the molecular pathways linking obesity to insulin resistance. Its utility extends to studying adipose tissue dysfunction, hepatic lipid metabolism, and immune-mediated inflammation—key drivers of T2DM pathogenesis.
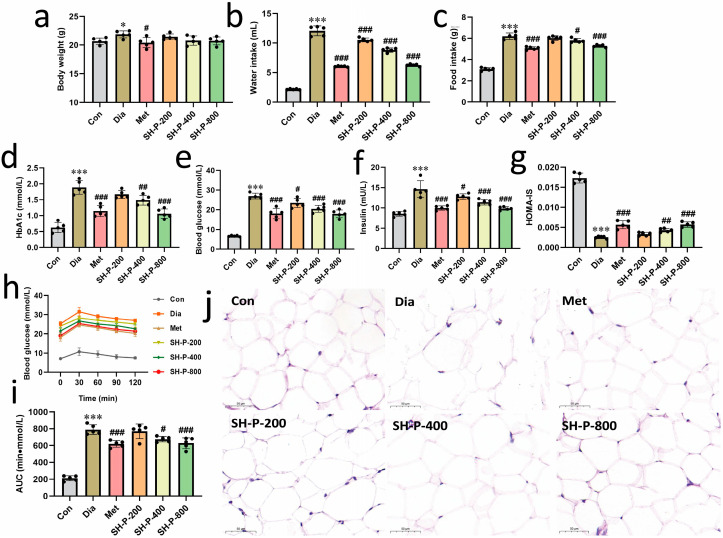 Fig.1 Improving hyperglycemia in T2DM mice with SH-P treatment.1,2
Fig.1 Improving hyperglycemia in T2DM mice with SH-P treatment.1,2
Applications of the C57BL/6J-HFD Model
The versatility of the C57BL/6J-HFD model has expanded its use across multiple research domains. In drug discovery, it serves as a preclinical platform for testing novel hypoglycemic agents and lipid-lowering therapies. Unlike acute models, its prolonged disease course allows assessment of long-term efficacy and safety, the latter being critical for metabolic drugs.
Complication research represents another frontier. The model recapitulates diabetic nephropathy, gastroenteropathy, and skeletal fragility, enabling investigations into tissue-specific damage mechanisms. For instance, studies have explored how chronic hyperglycemia and lipotoxicity contribute to renal fibrosis or bone density loss, insights that inform translational strategies.
Advancements in multi-omics technologies have further amplified the model's value. Integrating transcriptomic, proteomic, and metabolomic analyses allows identification of novel biomarkers and therapeutic targets. For example, liver transcriptomics has uncovered dysregulated pathways in fatty liver disease, while plasma metabolomics has identified lipid species associated with insulin resistance, bridging basic science to clinical diagnostics.
Service you may interested in
SEO Optimization Strategies for Academic Content
While SEO is typically associated with digital marketing, strategic keyword use can enhance the discoverability of academic publications. Primary keywords should include "diet-induced type 2 diabetes in mice," "C57BL/6J mice diabetes model," and "high-fat diet and diabetes," reflecting the study's focus. Secondary keywords like "type 2 diabetes mouse model" broaden search visibility without diluting specificity.
Title structure matters: placing keywords early, such as "Diet-Induced T2DM in C57BL/6J Mice," improves indexing by academic databases. The meta-description should succinctly summarize the model's advantages and comparative insights, balancing keyword inclusion with readability. Internal linking within multi-part publications can cross-reference sections on model validation and applications, enhancing logical flow and search engine crawling.
If you want to learn more about the diabetic animal models, please refer to:
Challenges and Future Directions
Despite its merits, the C57BL/6J-HFD model faces challenges. The long induction time (8–20 weeks) can hinder rapid research cycles, while inter-individual variability in HFD response may require larger sample sizes. Genetic background also plays a role, as C57BL/6J substrains or housing conditions can influence metabolic outcomes, necessitating standardized protocols.
Emerging technologies offer solutions. CRISPR-modified C57BL/6J strains allow targeted gene manipulation, enabling researchers to probe the role of specific genes in diet-induced T2DM. For example, knocking out inflammatory genes in adipose tissue can clarify their contribution to insulin resistance. Such precision models bridge the gap between observational studies and mechanistic insights.
Translational potential remains a key focus. Efforts to align rodent findings with human clinical trials include integrating humanized tissues (e.g., pancreatic islets) into C57BL/6J-HFD mice or using omics data to identify conserved pathways. These approaches aim to reduce translational gaps and improve the predictive value of preclinical research.
The diet-induced C57BL/6J mouse model has revolutionized T2DM research by offering a physiologically relevant platform to study disease progression from obesity to insulin resistance. Its ability to mimic early-stage human T2DM, combined with genetic tractability, makes it indispensable for mechanistic studies and preclinical drug development. As research evolves toward personalized and precision medicine, standardizing model protocols and fostering interdisciplinary collaboration will be crucial to unlock its full potential. By addressing current limitations through technological innovation, the C57BL/6J-HFD model is poised to remain a cornerstone of metabolic research, driving discoveries that translate to improved human health.
References
- Ni, Zaizhong, et al. "Phellinus igniarius polysaccharides ameliorate hyperglycemia by modulating the composition of the gut microbiota and their metabolites in diabetic mice." Molecules 28.20 (2023): 7136. https://doi.org/10.3390/molecules28207136
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
