What Should the DMPK Look Like in the Future?
DMPK offers critical foundations for assessing the drugability of candidate compounds via testing how drugs are absorbed, distributed, metabolized, and excreted within the human body. Traditional DMPK research is highly dependent on animal experiments. However, problems such as long experimental cycles, high costs, and significant species differences have always been bottlenecks restricting the efficiency of new drug research. Today, artificial intelligence (AI) and organ chips are driving the transformation of DMPK research from "experience-driven" to "precise prediction", ushering in a new era of "animal-free experiments".
In Silico DMPK: AI-driven digital drug metabolism prediction
1. Accurate prediction of metabolic sites based on structure
In Silico DMPK (computer simulation of drug metabolism and pharmacokinetics) uses AI algorithms to build a "digital pharmacist"-like predictive capability. Simply input the compound structure, and the model can predict the action site of metabolic enzymes (such as the CYP450 family) by analyzing characteristics such as chemical bond stability and electron cloud distribution. For example, AI can accurately identify easily oxidized carbon atoms on aromatic rings, hydrolysis sites of ester bonds, and even predict the probability of occurrence of binding reactions such as glucuronidation. A multinational pharmaceutical company used a deep learning model to predict the metabolic sites of 2,000 candidate compounds, with a degree of agreement with actual experimental results of 85%, which is more than 10 times more efficient than traditional empirical judgment.
2. Full chain simulation from molecular structure to in vivo exposure
The AI model can not only predict metabolic pathways, but also simulate the entire process of drug absorption from oral absorption, first-pass effect in the liver to systemic distribution by integrating the physiological pharmacokinetic (PBPK) model. Taking intestinal absorption as an example, the model can predict the efficiency of the compound's permeation through intestinal epithelial cells by calculating parameters such as the compound's lipid solubility (logP) and polar surface area (PSA); for the first-pass effect in the liver, AI can combine data such as liver blood flow and metabolic enzyme expression to accurately estimate the proportion of the drug entering the systemic circulation. In the development of a certain antidepressant, the AI model predicted in advance that its oral bioavailability was only 30%, prompting the R&D team to optimize the dosage form in a timely manner and avoid failure in the clinical stage.
Service you may interested in
Machine learning: giving DMPK predictions "data intelligence"
1. Insights into structure-metabolism relationships using millions of datasets
By training machine learning on DMPK data (such as clearance, half-life, and tissue distribution) of more than 100,000 known compounds, the AI model can capture the association between molecular features and metabolic behavior that are difficult to detect with traditional methods. For example, aromatic rings containing fluorine atoms are generally believed to enhance metabolic stability, but AI has found through analysis of clinical data that fluorine substitutions at specific positions may induce overexpression of the CYP3A4 enzyme and increase the risk of hepatotoxicity. The identification of such "exceptions" allows researchers to avoid potential risks during the compound design stage.
2. Accurate prediction and application of clinical metabolic typing
In a clinical trial of a certain antihypertensive drug, the patients will be divided into "fast metabolizers" and "slow metabolizers" by the AI model, the accuracy can up to 90%, and recommended personalized doses accordingly: fast metabolizers needed to increase their medication dosage by 20%, while slow metabolizers needed to reduce it by 30%, increasing the drug's effectiveness from 65% to 89% and reducing the adverse reaction rate by 40%. This "precision dosage" model is becoming an important direction for DMPK to extend to clinical precision medicine. Based on more than 100,000 clinical patients' genotypes (such as CYP2D6 gene polymorphism) and drug metabolism data, the AI model can predict individual metabolic capacity.
Organs on a Chip: A New Paradigm for DMPK Research in Miniature Human Bodies
1. Liver chip: the core of metabolic research to replace animal experiments
From the development history, as shown in the figure 1, liver chip technology has evolved from 2D single culture, 3D co-culture, to the use of bioreactors, 3D printing and tissue engineering technology, gradually improving physiological relevance and approaching animal models. Cultivating primary human hepatocytes in microfluidic chips and constructing a bionic blood flow system can highly simulate the metabolic microenvironment of the human liver. The principle is that microfluidic technology can accurately control the flow of liquids to provide liver cells with a nutrient supply and metabolic waste discharge environment similar to that in the body, and the bionic blood flow system makes the fluid dynamics characteristics in the chip similar to the microcirculation of the human liver.
Experimental data show that the metabolic rate of drugs in liver chips is 90% consistent with that of human liver, which can replace 80% of rat metabolic experiments. Taking the development of a lipid-lowering drug as an example, the liver chip detected that its metabolite M5 can cause mitochondrial damage in hepatocytes, while traditional rat experiments did not find this toxicity due to differences in metabolic enzymes. This is because the subtypes and expression levels of metabolic enzymes such as cytochrome P450 in rats are different from those in humans, and cannot accurately simulate the human body's metabolic process of drugs. This discovery allowed pharmaceutical companies to terminate the project in a timely manner, avoiding hundreds of millions of dollars in losses that might be incurred in the clinical stage. In the future, as technology develops, liver chips can also further improve the simulation of liver microenvironment by introducing more liver-related cell types (such as hepatic stellate cells, etc.), and are expected to replace more animal experiments, and play a greater role in liver disease modeling, drug hepatotoxicity assessment, disease diagnosis, and personalized treatment development (as shown in the figure 1 b).
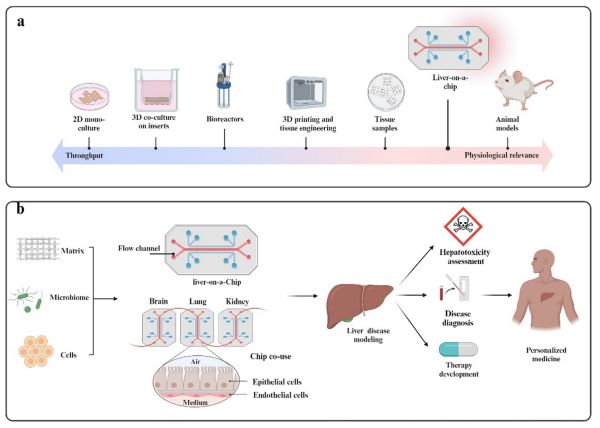 Fig. 1 Schematics of the development and application of Liver-on-a-Chip (LOC). 1,6
Fig. 1 Schematics of the development and application of Liver-on-a-Chip (LOC). 1,6
2. Blood-brain barrier chip: breaking through the "barrier" of central drug development
The blood-brain barrier (BBB) is composed of brain capillary endothelial cells (EC), pericytes (PC), and astrocytes (AC) end feet (as shown in the figure 2 a), which plays a role in protecting the brain and restricting the entry and exit of substances. The blood-brain barrier chip (BBB-on-a-chip) simulates its function through specific design. As shown in the second figure b, in the chip, the "blood" and "brain" areas are separated by a porous membrane to simulate the in vivo environment, and fluid flow channels and transepithelial electrical resistance (TEER) electrodes are set to monitor the barrier function. Its design is diverse, including two-chamber vertical chips, two-chamber horizontal chips and tubular designs (as shown in the figure 2 c).
In the anti-Alzheimer's disease drug test, the permeability of a candidate compound in the chip was only 5%, far lower than the 20% predicted by animal experiments. This is because the structure and function of the blood-brain barrier of animals are different from those of humans. For example, the expression and distribution of tight junction proteins in the blood-brain barrier of rodents are different from those of humans, resulting in inaccurate predictions of drug permeability. This result prompted the R&D team to develop a nanoliposome brain-targeted delivery system. The nanoliposome has good biocompatibility and targeting, and can cross the blood-brain barrier by interacting with brain vascular endothelial cells through receptor-mediated endocytosis. Ultimately, the drug concentration in the brain was increased by 10 times, and preclinical experiments showed that its clearance efficiency of β-amyloid protein was increased by 35%. Subsequent studies can further optimize the formulation and preparation process of nanoliposomes, improve their stability and drug loading efficiency, and explore the combined modification of multiple targeting ligands to enhance the targeting of diseased cells in different brain regions..
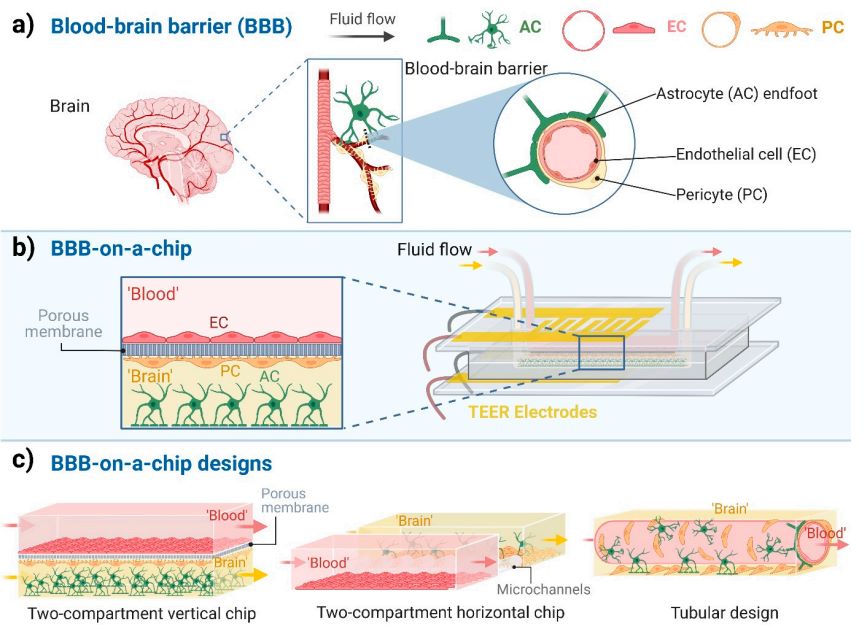 Fig. 2 Blood-brain barrier-on-a-chip models. 2,6
Fig. 2 Blood-brain barrier-on-a-chip models. 2,6
3.Lung Organ Chip: A "Living Model" to Revolutionize Respiratory Drug Development
As shown in the third figure, the lung organ chip simulates the gas-liquid interface and alveolar-capillary barrier by co-culturing human lung epithelial cells, endothelial cells and fibroblasts on a porous membrane chip, accurately reproducing the gas exchange, drug absorption and immune response process in the lung. The construction of the gas-liquid interface is the key. The special microfluidic design allows the cells to contact the gas on one side and the culture fluid on the other side to simulate the physiological environment in the alveoli. The porous membrane allows the cells to exchange substances and simulate the alveolar-capillary barrier function.
Experiments show that the drug deposition pattern in the chip is as consistent as 85% with the human lung CT scan data, which can effectively predict the distribution efficiency of inhaled drugs in the airways and alveoli. In the development of a new asthma inhaler, the lung organ chip not only captured the adhesion and retention of drug particles in the mucus layer, but also detected the apoptosis of type II alveolar cells caused by surfactant components. This is because the lung organ chip can monitor the changes in the physiological state of cells in real time, and can keenly capture the adverse effects of drugs on cells through cell viability detection, inflammatory factor secretion detection and other means. This discovery prompted the company to re-optimize the drug formula. The improved formula increased the drug penetration rate of the chip model by 40%, successfully shortened the preclinical development cycle by 18 months, and significantly reduced development costs. In the future, the lung organ chip can be combined with 3D bioprinting technology to construct a more complex lung tissue structure, further improve the simulation accuracy of lung disease models, and provide strong support for the research and development of more drugs and devices for the treatment of lung diseases.
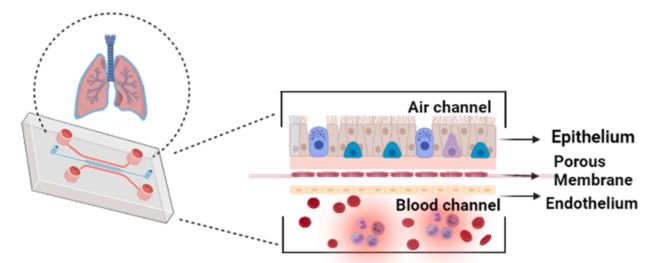 Fig. 3 Schematic of lung on chip portraying two sections namely-air channel and blood channel which mimics the human lung. 3,6
Fig. 3 Schematic of lung on chip portraying two sections namely-air channel and blood channel which mimics the human lung. 3,6
No animal testing: a technological revolution that brings both ethics and efficiency
1. Diversified breakthroughs in in vitro models
3D cell spheroid model: Through three-dimensional culture of tumor cells, it simulates the drug penetration and metabolic microenvironment of solid tumors, and has been used to study the distribution of anticancer drugs in tumor tissues, with a data correlation of 75% with clinical biopsy samples.
Organoid model: "Mini liver" and "Mini kidney" cultured with stem cells retain the three-dimensional structure and function of the organs, and their drug metabolism data are 70%-80% consistent with the human body. In the development of a liver disease drug, liver organoids successfully predicted the risk of drug-induced cholestasis, which was not detected by traditional 2D cell experiments.
2. Industry transformation driven by regulations
The European Union has clearly stipulated that animal experiments will be completely prohibited in DMPK research of cosmetic raw materials from 2025, and in vitro models must be used; the US FDA is accelerating the evaluation of the approval value of organ chip data. In 2024, the first anti-hepatitis drug based on liver chip data was approved, becoming a milestone case of "no animal experiments" to support the launch of new drugs. China's NMPA has also added a new approval clause for in vitro alternative models in the "Quality Management Standards for Non-clinical Drug Research" to promote the industry's transformation to green research and development.
Future trends: Three major technical directions reshape DMPK research
1. Real-time monitoring: from "post-mortem analysis" to dynamic tracking
The combination of near-infrared fluorescent labeling technology and micro-spectrometer will realize real-time monitoring of drug concentrations in living animals (such as mice). The frequency of data collection has been increased from "once a day" in traditional methods to "once a minute", which can capture instantaneous changes in drug metabolism-for example, a certain antibiotic reaches its peak kidney concentration 15 minutes after administration. This dynamic data provides an accurate basis for optimizing the dosing regimen and has more clinical guidance significance than traditional pharmacokinetic curve analysis.
2. Multi-omics integration: from a single dimension to system analysis
Combining multi-omics data such as metabolomics, proteomics, and transcriptomics with DMPK models can reveal the systemic effects of drugs on the body. In a study of a diabetes drug, multi-omics analysis unexpectedly found that its metabolites can activate PPARγ receptors and regulate lipid metabolism. This discovery prompted pharmaceutical companies to develop new indications (non-alcoholic fatty liver disease) and expand drug application scenarios.
3. Individualized DMPK: From group research to "one model for one person"
Based on individualized data such as patient genetic information (such as drug metabolizing enzyme genotype), intestinal flora composition, liver and kidney function indicators, a dedicated DMPK calculation model is constructed. Clinical experiments show that the individualized model for patients with depression can accurately predict the blood concentration of sertraline, with an error rate of less than 10%, which is significantly higher than the group model (error rate of 35%), providing technical support for the realization of "individualized dose".
The future of DMPK is the future of deep integration of AI algorithms, organ chips, and multi-omics technologies. From "virtual screening" of computer simulation to "in vitro verification" of organ chips, and then to "clinical transformation" of individualized models, these innovations are building a full-chain animal-free research and development system. With the maturity of technology and the improvement of regulations, DMPK will not only be a "navigator" of drug research and development, but also a "designer" of precision medicine, accelerating the birth of safe and effective drugs and opening up a new journey for human health.
If you want to learn more about the DMPK, please refer to:
References
- Liu, Jie, et al. "Construction of in vitro liver-on-a-chip models and application progress." BioMedical Engineering OnLine 23.1 (2024): 33. https://doi.org/10.1186/s12938-024-01226-y
- Kincses, András, et al. "The use of sensors in blood-brain barrier-on-a-chip devices: Current practice and future directions." Biosensors 13.3 (2023): 357. https://doi.org/10.3390/bios13030357
- Singh, Deepanmol, et al. "Journey of organ on a chip technology and its role in future healthcare scenario." Applied Surface Science Advances 9 (2022): 100246. https://doi.org/10.1016/j.apsadv.2022.100246
- da Silva, Renan Gonçalves Leonel, and Alessandro Blasimme. "Organ chip research in Europe: players, initiatives, and policies." Frontiers in Bioengineering and Biotechnology 11 (2023): 1237561. https://doi.org/10.3389/fbioe.2023.1237561
- Baillie, Thomas A. "Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism." Chemical research in toxicology 21.1 (2008): 129-137. https://doi.org/10.1021/tx7002273
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
