NZB/NZW F1 Mice: The Best Choice for SLE Research
In medical research, there is a kind of seemingly ordinary laboratory mice, but they carry the important mission of solving the mystery of human autoimmune diseases. They are NZB/NZW F1 hybrid mice, also known as BWF1 mice. How do these little creatures become "living models" for studying systemic lupus erythematosus (SLE)? In this article, I will analyze their origins, characteristics, and scientific research value.
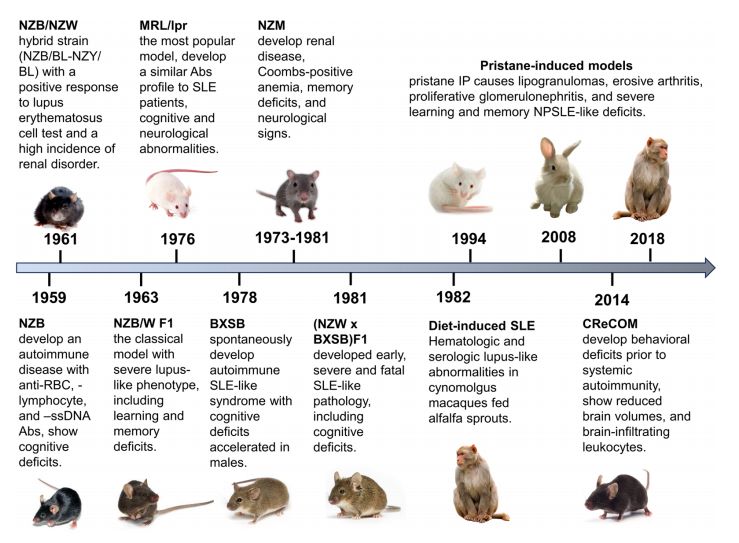 Fig. 1 Rogression and key manifestations of animal lupus models: a timeline overview.1,5
Fig. 1 Rogression and key manifestations of animal lupus models: a timeline overview.1,5
NZB and NZW mice: carriers of innate immune abnormalities
If you want to understand the magic of BWF1 mice, you must first trace the origins of its "parents" - the NZB and NZW inbred mice. As early as the 1940s, researchers discovered a special strain of mice in New Zealand, the NZB mouse (New Zealand Black). Subsequent observations revealed that these mice spontaneously developed hemolytic anemia after entering old age, and antibodies that attacked their own red blood cells could even be detected in their serum. This phenomenon showed that there must be something wrong with their immune system, and they couldn't even tell their own people apart! Later in-depth research also found that the B lymphocytes in the NZB mice were particularly "hyperactive" and would produce antibodies that attacked their own tissues at any time. This genetic characteristic makes them a natural material for studying autoimmune diseases.
Unlike the gray-brown coat of NZB mice, NZW (New Zealand White) mice are characterized by white coats and also originated in New Zealand. NZW mice do not show obvious autoimmune symptoms, but their immune system has defects in tolerance to self-antigens. The study found that NZW mice have abnormal T lymphocyte regulation function, especially insufficient regulatory T cells (Treg), which make it difficult to effectively suppress over-activated immune responses. This "mild" immune regulation defect laid the groundwork for the more complex autoimmune phenotype after hybridization with NZB mice.
As inbred strains of mice, NZB and NZW have highly homozygous genetic backgrounds, which means that mice of the same strain are almost completely identical at the genetic level. This feature makes the experimental results highly reproducible, making them ideal medical research models. When scientists crossbred these two strains of mice with different immune deficiencies, a "storm" of the immune system quietly brewed in their offspring.
Service you may interested in
The birth of BWF1 mice: an immune abnormality combination of 1+1>2
The first generation of offspring produced by the cross between NZB male mice and NZW female mice is NZB/NZW F1 mice, or BWF1 mice for short. This cross is not a simple gene superposition, but rather triggers a "synergistic abnormality" in the immune system. The BWF1 mouse strain is particularly interesting because it perfectly inherits the "problems" of its parents: the B cell hyperactivation characteristic from the NZB strain is "put together" with the T cell regulation defect from the NZW strain. These two defects are simply a "match made in heaven" (or a "lethal combination"), and the result is that when BWF1 mice grow to 3-4 months old, they will become ill without human intervention, showing symptoms very similar to human systemic lupus erythematosus (SLE). The most obvious sign is the presence of a large number of anti-nuclear anti-(ANA) in their blood. These ANAs are not good things. They can specifically trouble the components in the cell nucleus, such as DNA, histones, etc., and then combine with them to form so-called "immune complexes." The problem is that these complexes wander around in the blood and easily deposit in important organs, such as the kidneys, skin, and joints, and wherever they are placed, they will trigger an inflammatory storm. BWF1 mice develop proteinuria at 5-6 months of age, and immune complex deposits can be seen in the glomeruli, which proves the occurrence of lupus nephritis. The figure 1 shows the proteinuria level of n NZB/W F1 mice. Not only that, some mice also have facial erythema and arthritis. Are they similar to the clinical manifestations of human SLE patients?
Dig deeper from the immune mechanism: The B cells in BWF1 mice not only increase in number, but more importantly, they are "off track" - there is a problem with the differentiation and maturation pathway, like an out-of-control factory, continuously producing a large number of high-affinity autoantibodies. At the same time, the T cells that should be watching over these B cells also "strike", and are completely unable to effectively inhibit the proliferation and spread of B cells that specifically attack their own tissues. To make matters worse, the interferon signaling pathway in these mice is also abnormally active, which is like adding fuel to the already fierce immune inflammatory response, further "amplifying" the situation. The superposition of this multiple immune abnormality makes BWF1 mice one of the most classic SLE animal models.
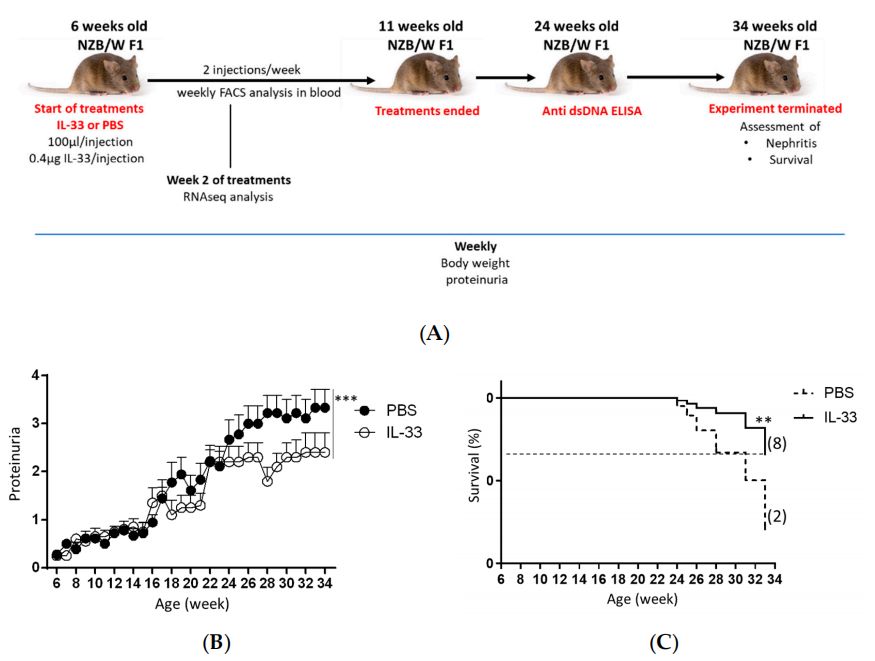 Fig. 2 Suppressed proteinuria level and mortality rate in NZB/W F1 mice treated with IL-3.2,5
Fig. 2 Suppressed proteinuria level and mortality rate in NZB/W F1 mice treated with IL-3.2,5
Heavy chain sequencing: decoding the molecular code of BWF1 mouse autoantibodies
In the study of BWF1 mice, antibody heavy chain sequencing technology plays a key role. The heavy chain gene of an antibody is highly diverse, and its sequence directly determines the specificity of the antibody's recognition of the antigen. In the BWF1 mouse model, scientists successfully tracked the evolution of these autoantibodies by sequencing the heavy chains of autoantibodies, thereby revealing the molecular mechanism of autoimmune response in systemic lupus erythematosus (SLE).
The study found that the heavy chain variable region (VH) of the antinuclear antibodies produced by BWF1 mice showed a clear preference for certain specific gene fragments. More importantly, the frequency of use of certain VH gene fragments is closely related to the pathogenicity of the antibody itself. A typical example is that autoantibodies that specifically attack double-stranded DNA (dsDNA) tend to prefer gene fragments such as VH11 or VH12. The amino acid sequences encoded by these fragments can accurately form specific binding structures with DNA molecules, which is the key to their driving the pathogenic process. Through heavy chain sequencing, scientists also found that the autoantibodies of BWF1 mice have undergone somatic high-frequency mutations, a process that should optimize the antibody's recognition of pathogens, but is mistakenly used to enhance the affinity for self-antigens in autoimmune diseases. These findings not only deepen the understanding of the pathogenesis of SLE, but also provide new ideas for targeted treatment. For example, immune intervention strategies targeting specific VH gene segments are expected to selectively inhibit the production of pathogenic autoantibodies without affecting normal immune function. Heavy chain sequencing technology is like a "detective tool" at the molecular level, allowing scientists to analyze the autoimmune "storm" of BWF1 mice at the genetic level, paving the way for precision medicine for human SLE.
Thymectomy experiment: exploring the regulatory role of T cells in SLE
The thymus is the "cradle" for the maturation of T cells. NZW mice with thymus removal (thymus ectomy) provide a unique model for studying the role of T cells in SLE. Experiments have found that after thymus removal in young NZW mice, their T cell development is blocked, especially the number of regulatory T cells is significantly reduced. When these mice (referring to the NZW strain) were crossed with NZB mice to produce BWF1 offspring, their autoimmune symptoms were often significantly aggravated and their survival was significantly shortened. This phenomenon confirms the central role of T cells, especially regulatory T cells (Tregs), in maintaining immune tolerance. Under normal circumstances, Treg cells act as the "brakes" of the immune system, effectively inhibiting over-activated autoreactive immune cells. Notably, when this group of cells was artificially removed through thymectomy, B cell activation in BWF1 mice became completely out of control: autoantibody production surged, and damage to organs such as the kidneys was exacerbated.
On the contrary, if normal regulatory T cells were injected into thymectomized BWF1 mice, their autoimmune symptoms would be significantly improved. These experiments not only verified the core position of the T cell regulatory network in the onset of SLE, but also provided experimental basis for cell therapy. At present, therapies based on regulatory T cells have entered the clinical trial stage of human SLE, and the thymectomy model of BWF1 mice has played an indispensable role in this research process.
BWF1 mice: a living platform to advance SLE research and drug development
As a standard animal model for SLE, BWF1 mice have irreplaceable value in drug development. From traditional immunosuppressants to new biologics, almost all candidate drugs for SLE need to verify their efficacy in BWF1 mice. For example, Belimumab is the first new biologic approved for SLE in recent decades. Its mechanism of action is to inhibit B cell activating factor (BAFF), and the discovery of this target is inseparable from the research in the BWF1 mouse model.
In BWF1 mice, scientists have also discovered many potential therapeutic targets. For example, overactivation of the type I interferon pathway is an important feature of SLE, and antibodies against interferon receptors can significantly alleviate symptoms in BWF1 mice; abnormal activation of the complement system is involved in the clearance of immune complexes, and complement inhibitors also show good renal protection in this model. These research results are gradually being transformed into clinical treatment plans to improve the prognosis of SLE patients.
In addition, BWF1 mice are also used to study the impact of environmental factors on SLE. For example, ultraviolet radiation can aggravate skin damage, simulating the photosensitivity of human SLE patients; viral infection (such as Epstein-Barr virus) can induce or aggravate autoimmune responses, providing a model for exploring the relationship between infection and autoimmune diseases.
Limitations and future developments of the BWF1 mouse model
Although BWF1 mice are a classic model for studying SLE, there are still differences between them and human diseases. For example, the renal lesions of BWF1 mice are mainly membranous lupus nephritis, while human SLE can manifest as more complex pathological types; in addition, mouse models cannot fully simulate the genetic background and environmental factors of humans. Therefore, scientists are trying to use gene editing technology to introduce human SLE-related genes into BWF1 mice to build a "humanized" model that is closer to human diseases.
With the development of new technologies such as single-cell sequencing and proteomics, the study of BWF1 mouse models has also entered the era of precision. By analyzing the heterogeneity of its immune cells at the single-cell level, scientists are expected to discover more sophisticated pathogenesis; combined with systems biology methods, mathematical models of SLE immune networks can be constructed to predict new therapeutic targets.
From the accidental discovery of NZB and NZW mice, to the systematic application of the BWF1 model, to the mechanism exploration of heavy chain sequencing and thymectomy experiments, these small experimental animals carry the hope of human victory over autoimmune diseases. They paved the way for medical research at the cost of their lives, allowing us to continue to move forward on the road to understanding SLE. Perhaps in the near future, research results based on the BWF1 mouse model can truly unveil the mystery of SLE and bring hope of cure to patients.
If you want to learn more about the NZB/NZW mice, please refer to:
References
- Polis, Baruh, Carla M. Cuda, and Chaim Putterman. "Animal models of neuropsychiatric systemic lupus erythematosus: deciphering the complexity and guiding therapeutic development." Autoimmunity 57.1 (2024): 2330387.https://doi.org/10.1080/08916934.2024.2330387
- Laurent, Laetitia et al. "Prevention of lupus nephritis development in NZB/NZW mice by selective blockade of CD28." European journal of immunology vol. 47,8 (2017): 1368-1376. https://doi.org/10.1002/eji.201746923
- Almizraq, Ruqayyah J et al. "(NZW × BXSB) F1 male mice: An unusual, severe and fatal mouse model of lupus erythematosus." Frontiers in immunology vol. 13 977698. 23 Sep. 2022, https://doi.org/10.3389/fimmu.2022.977698
- Mohd Jaya, Fatin Nurizzati et al. "Early Treatment of Interleukin-33 can Attenuate Lupus Development in Young NZB/W F1 Mice." Cells vol. 9,11 2448. 10 Nov. 2020, https://doi.org/10.3390/cells9112448
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
