Advances & Applications of Diabetic Animal Models
Introduction: The Significance and Classification of Diabetic Animal Models
Diabetes mellitus, a metabolic disorder affecting 537 million adults worldwide (WHO 2023), demands sophisticated animal models to unravel its pathophysiology. These models serve as indispensable tools for drug discovery, with 90% of preclinical diabetes studies relying on animal systems. Ideal models should replicate human disease features, including islet inflammation (T1D), insulin resistance (T2D), and microvascular complications.
Models are categorized by:
- Pathogenesis: T1D models (autoimmune/β-cell destruction) vs. T2D models (insulin resistance/β-cell exhaustion).
- Development: Spontaneous (genetic predisposition), induced (chemical/dietary), or engineered (gene editing).
- Species: Rodents (mice, rats) dominate due to low cost, while non-rodents (zebrafish, pigs) offer translational advantages for complex traits.
Construction and Characteristics of Major Diabetic Animal Models
Type 1 Diabetes (T1D) Models
- NOD Mouse: Derived from outbred Swiss mice, NOD mice carry MHC class II alleles (I-Ag7) that predispose to T-cell-mediated islet destruction. Disease progression involves CD4+ Th1 cell infiltration, IFN-γ-mediated β-cell apoptosis, and insulinopenia by 12–16 weeks. This model recapitulates human T1D's HLA-linked autoimmunity, making it pivotal for immunotherapy trials.
- STZ-Induced Model: STZ, a glucose analog, enters β-cells via GLUT2, generating reactive oxygen species that disrupt DNA. Single high-dose STZ (150–200 mg/kg) induces acute T1D in rodents within 72 hours, while low-dose protocols (40–60 mg/kg × 5 days) mimic slow β-cell attrition. Caution is needed: STZ impairs renal tubular function at ≥100 mg/kg.
Type 2 Diabetes (T2D) Models
- Zucker Diabetic Fatty (ZDF) Rat: The fa mutation in leptin receptor (LEPR) disrupts hypothalamic satiety signaling, leading to hyperphagia, obesity (400–600 g body weight), and hyperglycemia by 12 weeks. β-cell failure occurs via glucolipotoxicity, with islet amyloid deposition resembling human T2D.
- db/db Mouse: Homozygous Leprdb mutation abolishes leptin signaling, causing early-onset obesity (30–40 g at 8 weeks), insulin resistance, and nephropathy (mesangial expansion by 20 weeks). This model is ideal for testing SGLT2 inhibitors due to its severe glycemic dysregulation.
- HFD/STZ Combination Model: HFD (60% kcal from fat) for 8–12 weeks induces adipocyte hypertrophy and hepatic steatosis, followed by low-dose STZ (30–40 mg/kg) to impair β-cell mass. This protocol mirrors human T2D's "two-hit" hypothesis: insulin resistance precedes β-cell failure.
Specialized Models and Applications
- Gestational Diabetes Model: Pregnant rats fed HFD from gestational day 5, combined with STZ (20 mg/kg) on day 10, develop maternal hyperglycemia (≥11.1 mmol/L) and fetal macrosomia. This model mimics placental insulin resistance and fetal metabolic programming.
- Zebrafish Model: Larval zebrafish (72 hpf) exposed to 25 mM glucose show pancreatic β-cell loss, while crispr-mediated knockdown of insulin receptor (insr) causes persistent hyperglycemia. Their optical transparency allows live imaging of islet development using Tg(ins:GFP) transgenic lines.
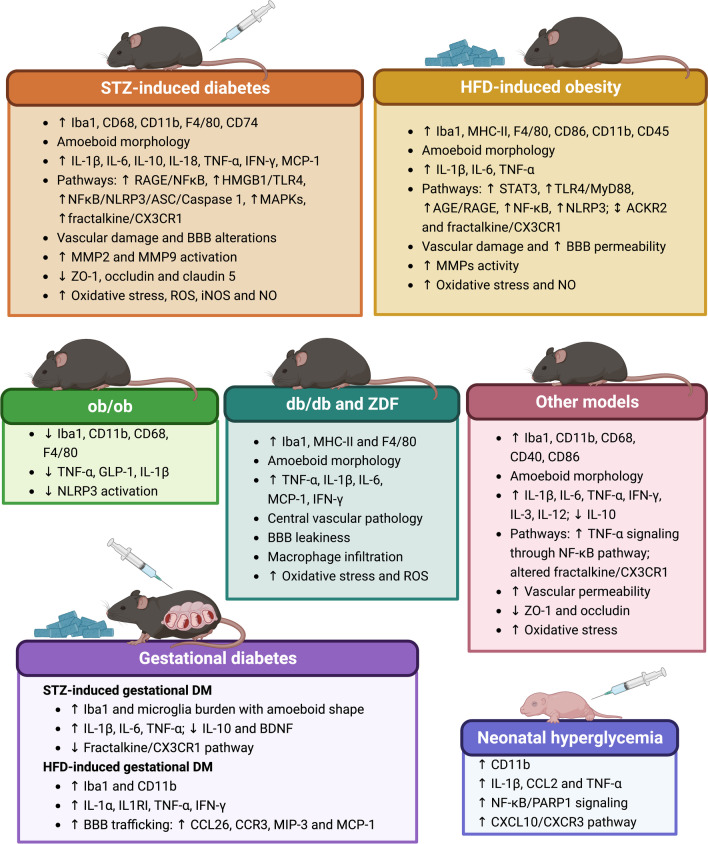 Fig.1 Microglial changes in preclinical diabetes.1.2
Fig.1 Microglial changes in preclinical diabetes.1.2
Model Applications in Key Research Domains
Diabetic Nephropathy
- db/db Mouse: By 16 weeks, these mice exhibit albuminuria (≥30 mg/g creatinine), glomerular basement membrane thickening, and TGF-β1-driven fibrosis. Studies here identified Sirtuin 1 (SIRT1) activation as a renoprotective mechanism.
- STZ-HFD Model: Combining STZ (40 mg/kg) with HFD for 16 weeks induces podocyte injury (nephrin loss) and tubular atrophy, suitable for testing ACE inhibitors (e.g., captopril) or GLP-1 receptor agonists.
- ZDF Rat: Advanced nephropathy (24 weeks) includes nodular glomerulosclerosis, a human-specific feature absent in mice. This makes ZDF rats valuable for evaluating novel antifibrotic agents like pirfenidone.
Obesity and Metabolic Syndrome
- ob/ob Mouse: Leptin deficiency leads to hypothalamic inflammation (microglial activation in arcuate nucleus) and dysregulated AMPK signaling in adipose tissue. These mice are used to study brown adipose tissue thermogenesis via UCP1 activation.
- KK-Ay Mouse: Carrying Ay allele of agouti gene, this model shows insulin resistance by 4 weeks, with hepatic steatosis driven by PPARγ dysregulation. It has been pivotal for understanding lipotoxicity-induced β-cell dysfunction.
Autoimmunity and Genetic Mechanisms
- NOD Mouse Immunoregulation: Regulatory T cell (Treg) dysfunction in NOD mice is linked to Foxp3 methylation defects. Adoptive transfer of Tregs from healthy mice delays diabetes onset, validating immune checkpoint targets.
- Genetically Engineered Models: Mice with β-cell-specific deletion of Irs2 (Irs2βKO) develop severe insulin resistance, while PPARγ haploinsufficiency (PPARγ+/-) impairs adipocyte differentiation, highlighting these pathways in T2D pathogenesis.
Service you may interested in
Advantages and Challenges in Model Selection
STZ Models vs. Genetic Models
- STZ Kinetics: Single-dose STZ models (e.g., C57BL/6 mice) achieve hyperglycemia within 3 days, ideal for acute drug efficacy tests (e.g., insulin sensitizers). Genetic models like NOD mice require 12–16 weeks for disease onset, suitable for chronic complication studies.
- Translational Limitations: STZ-induced models lack human-like islet autoimmunity, while genetic models (e.g., db/db) overexpress leptin receptor mutations not seen in most T2D patients.
Impact of Species Differences
Rodent vs. Human Nephropathy: Rodents rarely develop nodular glomerulosclerosis (K-W nodules), a hallmark of human diabetic nephropathy. Pigs, however, exhibit similar renal pathology when fed high-sucrose diets.
Zebrafish Metabolism: Zebrafish rely on hepatic gluconeogenesis (like humans) but lack brown adipose tissue, limiting thermogenesis research. Their pancreas lacks discrete islets, instead having diffuse endocrine cells.
Current Challenges
- Model Standardization: STZ potency varies by batch (Sigma-Aldrich vs. Santa Cruz), with pH adjustment (to 4.5–5.5) critical for β-cell specificity. Animal age also matters: 8-week-old rats are more STZ-sensitive than 12-week-olds.
- Complication Gaps: Most models fail to reproduce diabetic retinopathy (neovascularization) or neuropathy (myelinated fiber loss). Transgenic mice overexpressing VEGF show retinal angiogenesis but lack human-like capillary dropout.
- Translational Bottlenecks: PCSK9 knockout mice reduce LDL-C effectively, but clinical PCSK9 inhibitors show variable efficacy. This highlights species-specific lipid regulation differences.
Cutting-Edge Advances and Future Directions
Precision Model Development
CRISPR Multiplex Editing: CRISPR-Cas9 has created mice with concurrent mutations in T2D risk genes (e.g., TCF7L2, KCNJ11), recapitulating human polygenic inheritance. Such models show glucose intolerance not seen in single-gene knockouts.
Organoid-Transplanted Models: Human islet organoids derived from iPSCs, when transplanted into NOD-scid mice, form vascularized β-cell clusters that respond to glucose challenges. This system enables high-throughput screening of β-cell protectants.
Innovative Complication Studies
Zebrafish Nephropathy Screening: Transgenic zebrafish label podocytes, while Tg(lck:GFP) marks immune cells. High-content imaging quantifies albuminuria (via FITC-albumin) and inflammatory cell infiltration in 96-well plates.
Dynamic GDM Models: Miniature pigs fed a western diet during gestation show maternal hyperglycemia and fetal β-cell hyperplasia, tracked by PET-CT using 18F-FDG. Metabolomic analysis reveals fetal liver lipid dysregulation.
Interdisciplinary Technological Integration
AI-Powered Model Prediction: Machine learning algorithms (Random Forest) analyze 10,000+ model datasets to predict optimal modeling parameters. For example, an AI tool developed at Stanford University suggests HFD duration based on desired insulin resistance severity.
Spatial Transcriptomics: In db/db kidneys, spatial transcriptomics maps fibrotic niches to specific cell types (e.g., tubular epithelial cells expressing Col1a1). This guides targeted therapy development for diabetic nephropathy.
Conclusion: The Balancing Act in Model Selection
Effective diabetes research requires matching model traits to scientific goals: STZ models for rapid drug screens, genetic models for mechanistic depth, and non-rodents for translational validation. Emerging technologies, such as organoid transplantation and AI-driven optimization, promise to bridge the gap between bench and bedside. Yet rigorous validation against human disease remains essential, as no single model fully recapitulates diabetes' complexity. The future lies in integrative approaches combining multiple models to decode this multifactorial disorder.
If you want to learn more about the diabetic animal models, please refer to:
If you are in need of specific diabetic animal research models, please do not hesitate to get in touch with us. Our team of animal model experts is dedicated to understanding your unique research requirements and will craft a satisfactory solution tailored to your needs. Whether you are working on preclinical studies, drug development, or mechanistic research, our expertise ensures that you will receive reliable models and comprehensive support. Take the first step towards advancing your research–contact us today to discuss how our diabetic animal models can facilitate your scientific goals.
References
- Vargas-Soria, María, Mónica García-Alloza, and Miriam Corraliza-Gómez. "Effects of diabetes on microglial physiology: a systematic review of in vitro, preclinical and clinical studies." Journal of Neuroinflammation 20.1 (2023): 57. https://doi.org/10.1186/s12974-023-02740-x
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
