In Vitro ADME Screening: Accelerating Drug Development
In the complex landscape of pharmaceutical research, the failure rate of drug candidates remains a significant challenge. Historically, approximately 40% of drug attritions in late-stage development were attributed to poor absorption, distribution, metabolism, and excretion (ADME) properties. However, advancements in in vitro ADME screening have drastically mitigated this issue, reducing ADME-related failures to less than 10% in modern drug discovery pipelines. This shift underscores the critical role of ADME profiling in optimizing compound selection, minimizing costs, and accelerating timelines throughout the drug development lifecycle.
At its core, in vitro ADME screening serves as a cornerstone of modern pharmacology, enabling scientists to evaluate a compound's pharmacokinetic behavior early in the discovery process. By integrating high-throughput technologies and predictive models, this approach facilitates informed decision-making, ensuring that only compounds with favorable ADME properties progress to costly in vivo studies. This article explores the principles, applications, and future directions of in vitro ADME screening, highlighting its transformative impact on the drug discovery process, compound screening efficiency, and high-throughput drug development.
What is In Vitro ADME Screening?
Definition and Core Components
ADME, the fundamental pillars of pharmacokinetics, dictates how a drug interacts with the human body. In vitro ADME screening refers to the use of cell-based, tissue-derived, or synthetic models to evaluate a compound's behavior in four key domains:
- Absorption: How a drug enters systemic circulation (e.g., through the gastrointestinal tract).
- Distribution: The process of spreading throughout tissues and fluids.
- Metabolism: Enzymatic modification of the drug, typically in the liver.
- Excretion: The elimination of the drug or its metabolites from the body.
Unlike in vivo studies, which rely on animal models and human trials, in vitro screening offers controlled environments, reduced ethical concerns, and lower resource consumption. These assays provide mechanistic insights into compound behavior, allowing researchers to predict in vivo outcomes without relying on species-specific extrapolations.
Key Assays and Models
Modern in vitro ADME screening employs a diverse toolkit of assays to mimic physiological processes:
-
Permeability Assessment:
Caco-2 cell monolayers, derived from human colon carcinoma cells, replicate intestinal epithelium to evaluate oral absorption. Parallel artificial membrane permeability assays (PAMPA) offer a simplified, high-throughput alternative. Transporter panels, such as those targeting organic anion transporting polypeptides (OATPs) and organic cation transporters (OCTs), further refine predictions of active transport mechanisms. -
Metabolism Profiling:
Liver microsomes, rich in drug-metabolizing enzymes like cytochrome P450 (CYP450) and UDP-glucuronosyltransferases (UGTs), are used to assess metabolic stability and identify major clearance pathways. High-throughput enzyme inhibition assays help prioritize compounds with minimal interaction potential. -
Distribution Studies:
Protein binding assays, often using human plasma or serum, quantify a compound's affinity for albumin and α-1-acid glycoprotein, critical factors in determining free drug concentration. Tissue partitioning models estimate how drugs accumulate in specific organs or tissues. -
Excretion Modeling:
Renal clearance assays, using proximal tubule cell models, and hepatic clearance studies with hepatocytes or liver slices simulate elimination pathways, guiding predictions of half-life and dosing frequency.
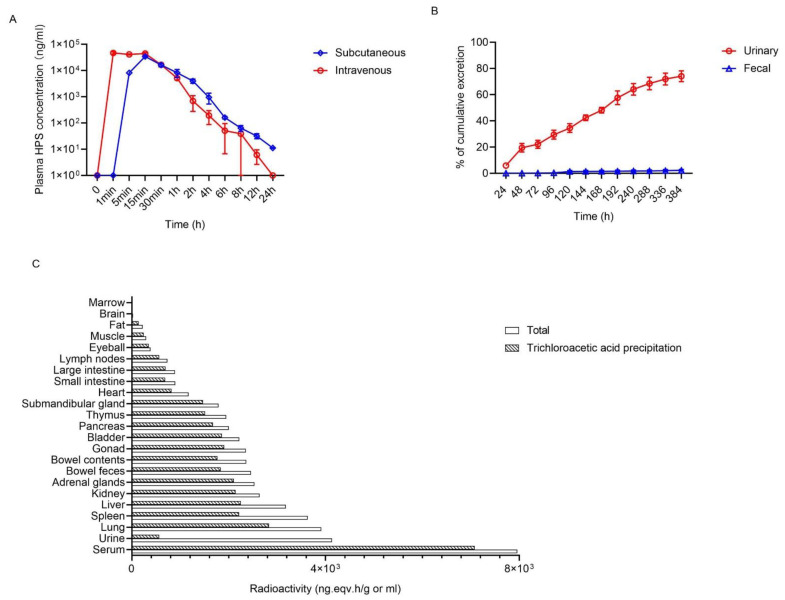 Fig.1 Pharmacokinetic profile of HPS. 1,2
Fig.1 Pharmacokinetic profile of HPS. 1,2
Service you may interested in
Role of ADME Screening in the Drug Discovery Process
Early-Stage Optimization
The primary value of in vitro ADME screening lies in its ability to eliminate non-viable compounds during the hit-to-lead phase. By prioritizing candidates with favorable permeability, metabolic stability, and low potential for drug-drug interactions, researchers avoid investing in compounds likely to fail in later stages. For example, high-throughput metabolic stability assays have been shown to reduce attrition rates by up to 50% in preclinical pipelines, significantly lowering the financial burden of advancing unsuitable molecules.
Safety and Efficacy Prediction
ADME screening also plays a pivotal role in risk assessment. Cytotoxicity assays and CYP inhibition studies flag potential safety concerns, while bioavailability simulations and half-life estimations inform dosing strategies. By integrating these data early, teams can optimize formulations to enhance therapeutic windows, balancing efficacy with tolerability.
Regulatory Compliance
Robust ADME datasets are essential for meeting regulatory requirements. The FDA and EMA increasingly emphasize comprehensive pharmacokinetic profiling in Investigational New Drug (IND) and New Drug Application (NDA) submissions. In vitro screening provides the mechanistic data needed to support these filings, streamlining review processes and reducing delays.
Traditional vs. Modern ADME Approaches
The evolution of ADME screening is marked by a shift from labor-intensive, low-throughput methods to automated, high-throughput systems. The table below contrasts key characteristics of traditional and modern approaches:
| Aspect | Traditional Methods | Modern ADME Screening |
|---|---|---|
| Throughput | Low (manual assays, limited scalability) | High (automation, 96/384-well plates, robotics) |
| Cost | High (reliance on animal/human trials) | Low (reduced reagent use, minimal animal models) |
| Predictive Accuracy | Moderate (species-specific limitations) | High (human-derived models, 3D cultures) |
| Regulatory Impact | Delays due to incomplete or inconsistent data | Faster IND approvals via robust, integrated datasets |
Modern technologies, such as automated liquid handling systems and label-free detection methods, enable simultaneous screening of thousands of compounds. Human-derived models, including induced pluripotent stem cell (iPSC)-derived hepatocytes, address the species mismatch inherent in traditional animal studies, improving translational relevance.
Challenges and Future Trends
Current Limitations
Despite advancements, in vitro ADME screening faces challenges. Two-dimensional (2D) cell cultures, while convenient, often fail to recapitulate the complexity of in vivo tissues, such as cellular heterogeneity and extracellular matrix interactions. Additionally, integrating data from diverse assays (e.g., permeability, metabolism, and toxicity) into a unified predictive model remains technically challenging, requiring sophisticated computational frameworks.
Emerging Innovations
-
Organ-on-a-Chip (OOC) Technology:
OOC models, such as liver and intestine chips, mimic organ physiology using microfluidic systems and primary human cells. These platforms enable dynamic, multi-cellular studies of drug absorption and metabolism, bridging the gap between in vitro and in vivo environments. -
AI-Driven ADME Prediction:
Machine learning and generative AI models are revolutionizing compound design. By analyzing vast datasets of ADME properties, these algorithms can predict metabolic stability, permeability, and toxicity of novel structures, accelerating the hit identification process. -
3D Cell Models:
Three-dimensional hepatocyte spheroids and organoids exhibit enhanced metabolic functionality compared to 2D cultures, providing more accurate assessments of drug clearance and toxicity.
Integration and Standardization
The future of ADME screening lies in integrating multi-omics data (genomics, proteomics, metabolomics) with high-throughput assays. Standardized protocols and shared databases will also enhance data reproducibility, fostering collaboration across the pharmaceutical industry.
In vitro ADME screening has evolved from a supplementary tool to a central driver of drug development. By enabling early identification of compounds with optimal pharmacokinetic profiles, this approach reduces attrition, cuts costs, and accelerates the journey from discovery to clinic. As technologies like organ-on-a-chip and AI-driven modeling mature, the field is poised to further enhance predictive accuracy and streamline workflows.
For the pharmaceutical industry, embracing these innovations is not just a matter of efficiency—it is essential for staying competitive in an era of precision medicine. By prioritizing integrated, high-throughput ADME screening, researchers can ensure that the next generation of therapies is safer, more effective, and brought to market faster than ever before.
If you want to learn more about the transgenic mice, please refer to:
- Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
- In Vitro ADME Assays: Principles, Applications, and Protocols
- Differences and Connections Between ADME and DMPK in Drug Research
- In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insight
- In Vitro ADME and In Vivo PK Studies: A Holistic Framework for Drug Evaluation
- ADME in Toxicology: Ensuring Drug Safety and Efficacy
When it comes to drug ADME testing, trust the expertise of a team with years of proven experience. At Creative Biolabs, we boast a deep understanding and extensive track record in delivering robust ADME testing solutions that provide authentic, reliable data to fuel your drug development journey. Our seasoned scientists leverage cutting-edge methodologies and rigorous protocols to ensure every dataset we generate is both accurate and actionable, empowering you to make informed decisions at every stage of development. Whether you're optimizing lead compounds, assessing pharmacokinetics, or mitigating potential risks, our reliable ADME insights serve as a cornerstone for accelerating your drug development pipeline. Don't leave the success of your research to chance—partner with a team that prioritizes data integrity and scientific excellence. Contact us today to discuss how our decades of ADME expertise can propel your drug discovery forward and turn challenges into breakthroughs!
References
- Yang, Yang, et al. "Recombinant human HPS protects mice and nonhuman primates from acute liver injury." International Journal of Molecular Sciences 22.23 (2021): 12886. https://doi.org/10.3390/ijms222312886
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
