Humanized Mice for Antibody Production & Disease Modeling
Humanized mice, created through genetic engineering or transplantation of human genes, cells, tissues, or organs, are vital tools bridging the gap between traditional animal models and human biological complexity. Their core purpose is to accurately simulate human physiology, immunology, and genetics within a murine system. This addresses the critical limitation of conventional models, which often fail to replicate human-specific mechanisms, leading to translational research gaps. By incorporating human components, these mice provide a significantly more relevant preclinical platform, enhancing research accuracy and accelerating biomedical discoveries.
The primary applications of humanized mice are in antibody development and disease modeling. For antibody production, they enable the generation of fully human antibodies, overcoming the immunogenicity problems associated with mouse-derived antibodies. In disease modeling, they allow researchers to create sophisticated models that faithfully mimic human diseases, facilitating essential mechanistic studies, drug screening, and toxicity testing. Overall, humanized mice are indispensable for advancing insights into human-specific responses to diseases and therapies, significantly impacting biomedical research progress.
Humanized Mice in Antibody Production
Overcoming Challenges in Traditional Monoclonal Antibody Production
Immunogenicity of Murine Antibodies: Traditional mouse-derived monoclonal antibodies often trigger immune responses in humans, reducing their efficacy and causing adverse effects.
Need for Fully Human Antibodies: The demand for human antibodies that evade immune detection has driven the development of humanized mouse models. These models allow for the production of antibodies that are genetically human, minimizing immunogenicity risks.
Strategies for Constructing Humanized Mouse Models
Genetic Engineering Techniques: Advanced methods like embryonic stem (ES) cell targeting and CRISPR-based gene editing enable the integration of human immunoglobulin gene loci (e.g., IgH, IgL) into the mouse genome. This replaces the murine antibody-producing genes with human counterparts, allowing the mice to generate human antibodies upon immunization.
Importance of Immunodeficient Mice: Strains such as SCID (Severe Combined Immunodeficiency) and Rag⁻/⁻ mice, which lack functional adaptive immune systems, serve as ideal hosts. Their immunodeficiency prevents rejection of human cells or tissues and ensures the humanized immune system can develop unhindered.
Advantages of Humanized Mice for Antibody Production
High-Affinity, Specific Human Antibodies: Humanized mice generate antibodies with human-like specificity and affinity for target antigens, crucial for therapeutic efficacy.
Reduced Immunogenicity in Clinical Use: By eliminating murine antibody components, humanized mice-derived antibodies minimize the risk of immune reactions in patients.
Accelerated Antibody Drug Development: The ability to rapidly screen and isolate human antibodies in vivo shortens the R&D cycle, reducing costs and time to clinical trials.
Application Examples
Antitumor Antibodies: Humanized mice have facilitated the development of antibodies targeting cancer antigens, such as those used in checkpoint immunotherapy.
Autoimmune Disease Therapies: Models have enabled the creation of antibodies that modulate immune responses in diseases like rheumatoid arthritis.
Infectious Disease Antibodies: Antibodies against pathogens like HIV and influenza have been developed using humanized mouse platforms, targeting human-specific viral entry mechanisms.
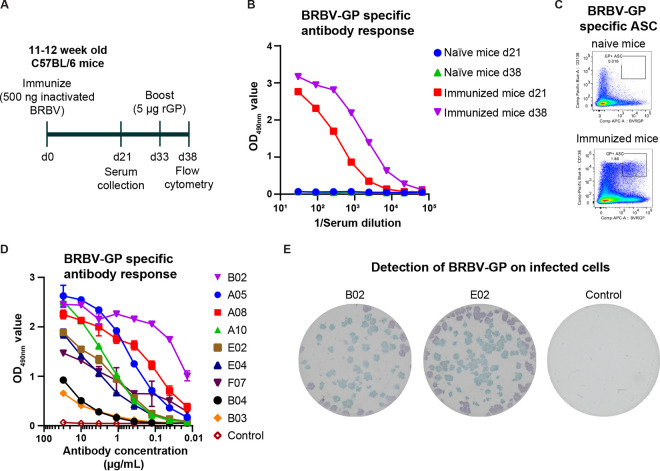 Fig.1 Humanized monoclonal antibody development against BRBV GP.1,2
Fig.1 Humanized monoclonal antibody development against BRBV GP.1,2
Service you may interested in
Humanized Mice in Disease Modeling
Needs and Challenges in Disease Modeling
Limitations of Traditional Animal Models: Rodent models often fail to replicate human disease pathophysiology, especially for conditions with complex genetic or immunological bases.
Demand for Physiologically Relevant Models: Humanized mice address this by recapitulating human-specific cellular, tissue, and organ functions, enabling more accurate disease simulation.
Construction Strategies for Humanized Mouse Models
Immune System Humanization
- Transplantation of Human Hematopoietic Stem Cells (HSCs): HSCs are introduced into immunodeficient mice, differentiating into a human-like immune system, including T cells, B cells, and myeloid cells.
- Transplantation of Human Peripheral Blood Mononuclear Cells (PBMCs): PBMCs, containing mature immune cells, are engrafted to rapidly reconstitute immunity, though with potential graft-versus-host disease (GvHD) risks.
- Humanized Lymphoid Organs: Co-transplantation of human thymus and liver tissues supports immune cell maturation, enhancing model fidelity.
Gene-Specific Humanization: Disease-related human genes are knocked into or out of the mouse genome, modeling genetic disorders like neurodegenerative diseases.
Tissue/Organ Humanization: Organs such as the liver or brain are replaced or supplemented with human tissues, enabling studies of human-specific organ functions and diseases.
Application Examples
Infectious Disease Models
- HIV/AIDS: Humanized mice with reconstituted immune systems support HIV infection studies, aiding antiviral drug and vaccine development.
- Viral Hepatitis (HBV, HCV): Humanized liver models allow hepatitis virus replication and testing of antiviral therapies.
- Other Infections: Models for bacterial (e.g., tuberculosis) and viral diseases simulate human pathogen-host interactions.
Cancer Models
- Patient-Derived Xenograft (PDX) Models: Tumor tissues from cancer patients are transplanted into humanized mice, preserving tumor heterogeneity and microenvironment.
- Tumor Microenvironment Simulation: Humanized immune cells within the tumor microenvironment enable studies of immunotherapy responses.
Autoimmune Disease Models
- Inflammatory Bowel Disease (IBD): Humanized immune systems drive gut inflammation similar to human IBD, supporting drug screening.
- Rheumatoid Arthritis: Models recapitulate joint inflammation and autoantibody production, aiding therapeutic development.
Neurodegenerative Disease Models
- Alzheimer's Disease: Humanized mice expressing mutant human amyloid or tau proteins develop amyloid plaques, mimicking neurodegeneration.
- Parkinson's Disease: Models with human α-synuclein gene mutations replicate dopaminergic neuron loss and motor dysfunction.
Drug Screening and Toxicity Assessment: Humanized models evaluate drug efficacy and safety in a human-relevant context, reducing reliance on animal-only data.
Comparison with Traditional Models and Future Challenges
Key Differences: Traditional vs. Humanized PDX Models
| Parameter | Traditional PDX Models | Humanized PDX Models |
|---|---|---|
| Modeling Time | Months to years | Days to weeks |
| GvHD Risk | Present (with immune cells) | Negligible |
| Immune Microenvironment | Limited (murine immune cells) | Full human immune system |
Traditional PDX models, while valuable, lack a human immune system and require lengthy establishment. Humanized PDX models, however, enable rapid modeling with intact human immunity, ideal for immunotherapy studies.
Existing Challenges
Functional Limitations: The murine cytokine environment may not fully support human immune cell functions, potentially affecting model accuracy.
Cost and Technical Barriers: Stem cell transplantation and maintenance of humanized mice involve high costs and specialized expertise, limiting accessibility for some researchers.
Ethical and Translational Considerations: Balancing scientific need with animal welfare concerns remains a priority, driving efforts to refine model efficiency.
Humanized mice have revolutionized biomedical research, offering unprecedented insights into human biology, disease mechanisms, and therapeutic responses. Their role in producing high-quality human antibodies and modeling complex diseases has accelerated drug development and improved translational outcomes. As technology advances—including more precise gene editing, improved immune reconstitution, and organoid-based humanization—these models will become even more integral to understanding and combating human diseases. While challenges in cost, functionality, and ethics persist, the future of humanized mice in antibody discovery and disease modeling is promising, holding the key to transformative medical breakthroughs.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Bamunuarachchi, Gayan, et al. "Detection of Bourbon virus-specific serum neutralizing antibodies in human serum in Missouri, USA." MSphere 7.3 (2022): e00164-22. https://doi.org/10.1128/msphere.00164-22
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
