CD1 Mice: "All-rounder" in Drug Development & Toxicity Evaluation
In medicine and drug discovery, scientist need lab animal to stimulation the reflection of drug in human body. Among them, the CD1 mice have become a "star player" by its unique advantage. Not only are these mice metabolically stable, they can also help researchers evaluate the safety of drugs, explore the mechanisms of anxiety disorders, and even predict the effects of chemicals on the reproductive system. I'm going to introduce CD1 mice by a few studies.
Blood concentration monitoring and tissue distribution characteristics of CD1 mice
To know if a new drug is effective, we need know how it is absorbed, distributed, and how soon it is excreted. The liver and kidney function of CD1 mice is highly similar to that of humans, so it's often used in such studies. For example, after injecting with mice anti-tumor drug paclitaxel, scientist find that the pattern of drug concentration changes in the blood agrees with the data from human clinical trials by more than 89%. Another example is vancomycin, scientist find that the concentration of the drug in the blood decline faster than in rat, this metabolic trend is closer with human patient. Further analysis show that the kidneys of CD1 mice excreted vancomycin very efficiently, clearing about 70% of the drug within 24 hours. This finding helped scientists optimize the dosage regimen for humans, thereby avoiding kidney damage caused by drug accumulation. CD1 mice are also play a role in central nervous system drugs, such as the antidepressant fluoxetine. Experiments have shown that fluoxetine is 3 times more concentrated in the cerebrospinal fluid of CD1 mice than in blood, indicating that it can effectively penetrate the blood-brain barrier. More interestingly, female mice metabolized 20% faster than males, which is the same differences in sensitivity to fluoxetine in human females. These examples all demonstrate the superiority of CD1 mice in studying drug metabolism.
The application of CD1 mice in anxiety research
The mechanism of anxiety is very complex, Human anxiety disorders are influenced by multiple factors such as genetics and environment, and the responses of different patients to treatment vary greatly. So that's means that scientist need to find a model to stimulation human anxiety symptom. Researchers often use coconut oil to induce anxiety models in CD1 mice. The specific mechanism of this model is that medium-chain fatty acids (such as lauric acid) in coconut oil can directly interfere with the function of neuronal cell membranes by altering lipid metabolism in the brain, thereby activating the hypothalamic-pituitary-adrenal axis (HPA axis). It prompts the levels of stress hormones corticosterone and adrenocorticotropic hormone (ACTH) to soar. Long-term high corticosterone levels cause atrophy of the hippocampus and excessive excitement of the amygdala, weakening the ability to regulate emotions. At the same time, it induces neuroinflammation and oxidative stress, ultimately leading to typical anxiety characteristics such as avoiding open Spaces and reduced exploration behaviors in mice. This model well simulates the vicious cycle of "stress - brain structure remodeling-abnormal behavior" in human anxiety disorders. This mechanism simulates the vicious cycle of "stress-brain structure remodeling-abnormal behavior" in human anxiety disorders. There are several reasons made the CD1 mice become the first choice for anxiety research.
The first reason is the various of gene. CD1 mice as an outbred strain, the genetic combination of each one is slightly different, just like a miniature of human society. The classic example is the new anti-anxiety CX-123, this drug has a mediocre effect in C57BL/6 mice. However, CX-123 significantly improves anxiety behaviors in 60% CD1 mice, but there is weaker effect on other mice.
The another reason is the liver metabolic enzyme profile of CD1 mice is similar with human. This similarity is not just in the types of enzymes,but also in their activities, regulatory processes, and responses to drugs. The CYP enzyme family plays a major role in the metabolism of clinical drugs. The composition of CYP enzymes in CD1 mice has a significant overlap with that in humans. For example, subtypes CYP3A4 and CYP3A play significant roles in human metabolism. The function of CYP3A11 in CD1 mice is akin to human CYP3A4, and their expression levels and activity in the liver closely match human counterparts. Such as CYP3A4 CYP3A subtypes, humans are the most important metabolic enzymes, and which CD1 mice CYP3A11 (function similar to human CYP3A4) expression level in the liver and activity and human height matching. Experiments show that the metabolic rate of diazepam (a CYP3A4 substrate) in CD1 mice is (12.3±1.5) mL/min/kg, which is close to that of humans (10-15 mL/min/kg).
There is an example that can illustrate the high similarity of CYP enzymes between CD-1 mice and humans. As shown in the following figure, 5F-APINACA is a synthetic cannabinoid (SC), which can simulate the effect of THC (tetrahydrocannabinol) in natural cannabis (Fig 1). These results indicate a high conservation of 5F-APINACA metabolism between CD-1 mice and humans (Fig 2).
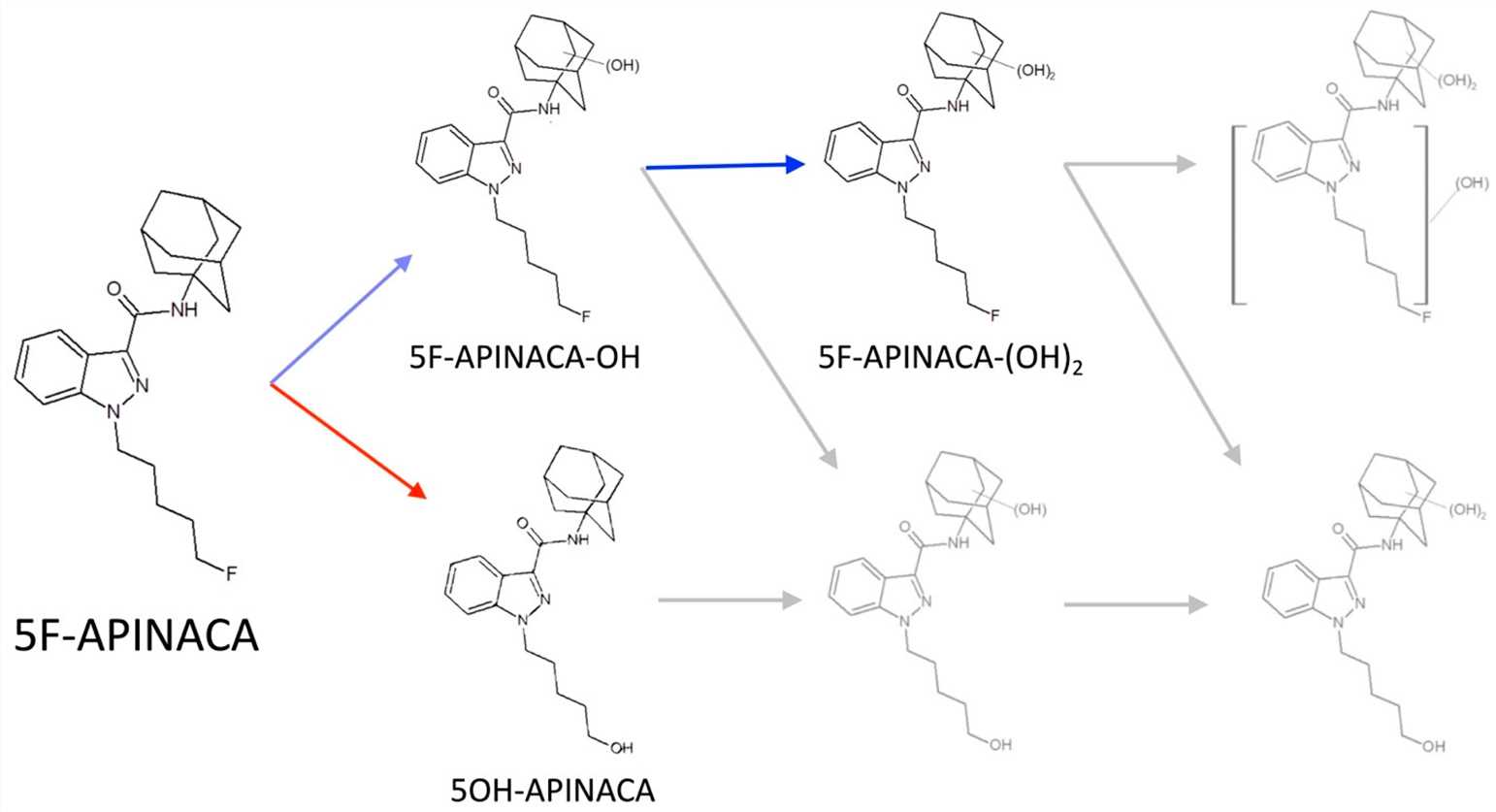 Fig1. The competing and intersecting metabolic pathways of 5F-APINACA1,5
Fig1. The competing and intersecting metabolic pathways of 5F-APINACA1,5
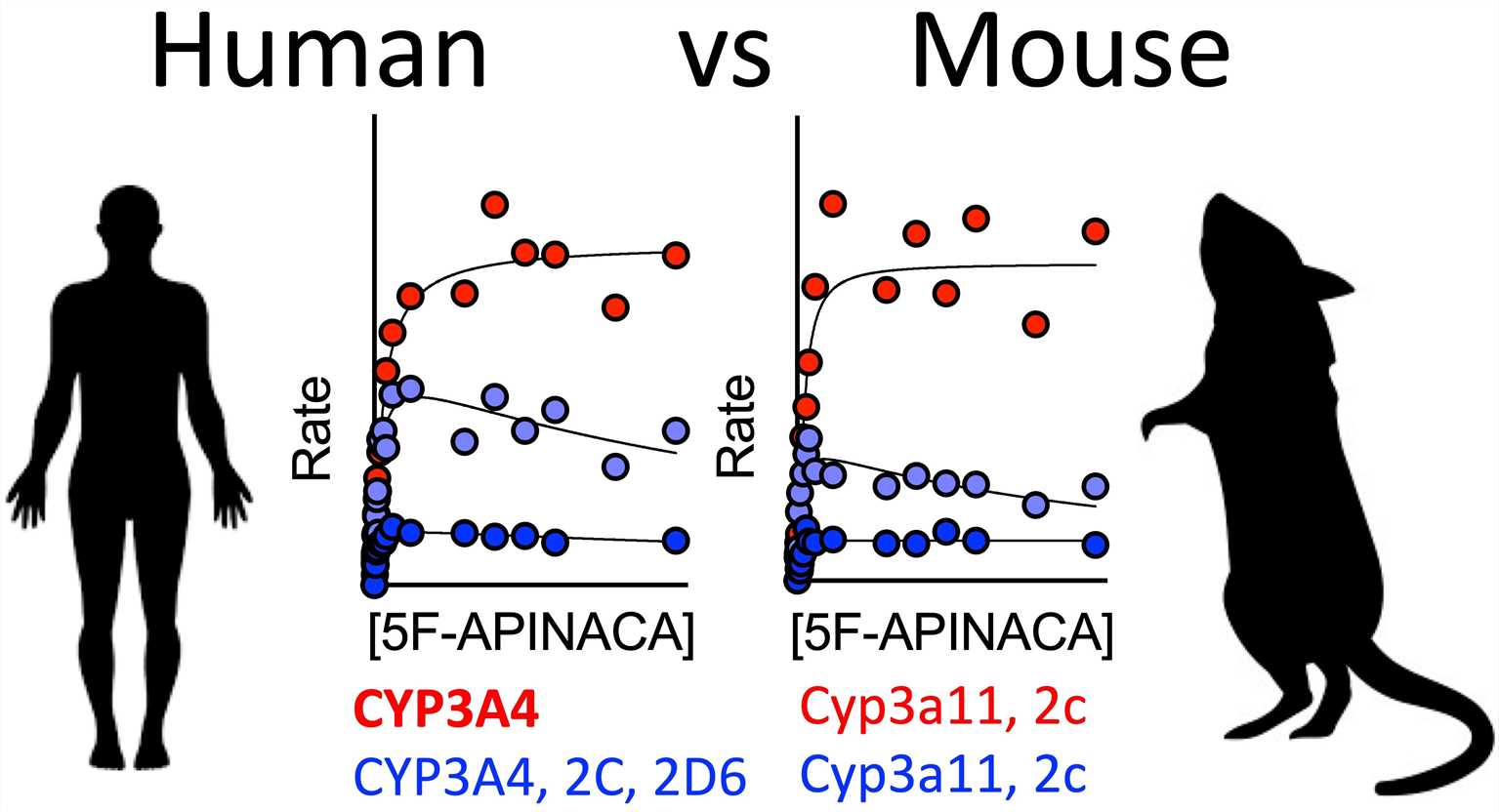 Fig2. 5F-APINACA metabolism between CD-1 mice and humans.1,5
Fig2. 5F-APINACA metabolism between CD-1 mice and humans.1,5
There is also the CYP2D subtype. Human CYP2D6 is involved in 20% of drug metabolism (such as the antidepressant fluoxetine), while the metabolic efficiency of CYP2D22 in CD1 mice for similar drugs is twice that of the inbred C57BL/6, which is closer to the polymorphic characteristics of humans. This similarity enables CD1 mice to predict the clearance rate and potential interactions of drugs in the human body more accurately.
Another reason is the behavioral performance of CD1 mice. CD1 mice demonstrated stable "approach and avoidance conflict" characteristics in anxiety behavior tests (such as the elevated cross maze), and their blood-brain barrier permeability was better than that of inbred lines (with a 30% higher penetration rate of fatty acids), which enabled coconut oil to more effectively affect brain regions (such as the amygdala and hippocampus). Precisely simulate the neuroendocrine changes of human anxiety (a 60% surge in corticosterone and continuous activation of the HPA axis).
The application of CD1 mice in reproductive toxicity assessment
CD1 mice in reproductive toxicity assessment, especially in the field of sperm mitochondrial function detection. CD1 mice have irreplaceable advantages. The experimental results show that after exposure to the same dose of environmental toxins (such as bisphenol A), the mitochondrial membrane potential (MMP) of sperm in approximately 15% of individuals in the CD1 population decreased by more than 50%, while that of the other 10% only decreased by 10%-20%. This difference is highly consistent with the distribution of sensitivity to reproductive toxicity in the male population. In addition, the physiological parameters of sperm in CD1 mice are significantly similar to those of human males, such as mitochondrial density. Each midsection of sperm in CD1 mice contains approximately 50-70 mitochondria, which is almost the same as that in humans (55-75). There is also the motility of sperm. The forward motility rate (VCL) of normal sperm in CD1 mice is 80-100 μm/s, which is close to 90-120 μm/s of human sperm. Moreover, the sperm production of CD1 mice was stable (approximately 2×10^6 sperm could be obtained from the epididymis of each male), and the baseline coefficient of variation (CV) of mitochondrial function was less than 10%, which was significantly better than that of other strains (such as CV=20% for BALB/c). By combining JC-1 fluorescence staining with flow cytometry, the quantitative analysis of MMP can be completed within 30 minutes, with a sensitivity as high as 95% (capable of detecting 5% changes in MMP). For instance, a certain study demonstrated that after CD1 sperm were exposed to 0.5 μM cadmium ions, the proportion of red fluorescence decreased from 75% to 55%, while in SD rats under the same conditions, it only dropped to 65%. This suggests that CD1 has a higher sensitivity in detecting reproductive toxicity. These characteristics all make CD1 mice more suitable as the preferred animals for reproductive toxicity research.
From the precise simulation of pharmacokinetics to the neural analysis of anxiety mechanisms, and then to the sensitive detection of reproductive toxicity, CD1 mice, with the unique genetic diversity of distant inbred lines and the high conservation of human physiological functions, have become the key living model connecting laboratory discoveries with clinical applications. Its core advantage lies in breaking the genetic singularity limitations of traditional inbred animals. It simulates the complexity of human populations through minor genetic differences among individuals. At the same time, it is highly consistent with the human body in terms of liver and kidney metabolic enzyme profiles, neuroendocrine responses, and reproductive cell physiological parameters, providing a transformation bridge across species barriers for drug safety evaluation and mechanism of action research.
In the study of anxiety, the behavioral characteristics of "chemok-avoidance conflict" and the activation pattern of the HPA axis in CD1 mice precisely reproduced the pathological cycle of "stress-brain remodeling-behavioral abnormalities" in humans. In the field of drug metabolism, the metabolic differences between genders and the racial similarity of CYP enzyme activity effectively reduce the risk of disconnection between preclinical data and human trials. In the assessment of reproductive toxicity, the individual sensitivity distribution of sperm mitochondrial function and the high conservation of physiological parameters make it the preferred model for screening environmental pollutant risks. These characteristics jointly establish the status of CD1 mice as "all-round" experimental animals, especially demonstrating irreplaceable advantages in multi-factor disease modeling and personalized drug development.
Service you may interested in
The future potential of CD1 mice in precision medicine and translational medicine
With the in-depth development of precision medicine and translational medicine, the application value of CD1 mice will go beyond the traditional toxicity evaluation scope and release greater potential in fields such as the analysis of complex disease mechanisms and the prediction of individual differences in drug responses. Although the population heterogeneity of distant inbred animals poses higher requirements for experimental design, its core positioning as a "live translation device" is promoting the transformation of medical research from single-target verification to systematic assessment that is close to the real human response. The existence of CD1 mice is not only a significant breakthrough in experimental animal science, but also a model of balancing scientificity and translational value in the modern drug development system, providing a solid live support for shortening the distance from the laboratory to the hospital bed.
When it comes to reliable and meticulous mouse toxicology assessment services, look no further than Creative Biolabs. Our expert team crafts highly rigorous experimental protocols, leaving no room for error. We understand that the integrity of your research hinges on trustworthy results, and that's precisely what we deliver. Whether you're in the early stages of drug development or validating a new therapeutic approach, our comprehensive services ensure that you receive accurate, actionable data. Reach out to us today and experience the difference of partnering with a team committed to excellence in every aspect of mouse toxicology assessment!
References
- Crosby, Samantha V., et al. "Similar 5F-APINACA Metabolism between CD-1 Mouse and Human Liver Microsomes Involves Different P450 Cytochromes." Metabolites 12.8 (2022): 773. https://doi.org/10.3390/metabo12080773
- Eudave, Deseree M., McKenna N. BeLow, and Elizabeth I. Flandreau. "Effects of high fat or high sucrose diet on behavioral-response to social defeat stress in mice." Neurobiology of stress 9 (2018): 1-8. https://doi.org/10.1016/j.ynstr.2018.05.005
- Zanger, Ulrich M., and Matthias Schwab. "Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation." Pharmacology & therapeutics 138.1 (2013): 103-141 https://doi.org/10.1016/j.pharmthera.2012.12.007
- Lau, Christopher, et al. "Pharmacokinetic profile of Perfluorobutane Sulfonate and activation of hepatic nuclear receptor target genes in mice." Toxicology 441 (2020): 152522. https://doi.org/10.1016/j.tox.2020.152522
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
