DMPK Bioanalysis
DMPK bioanalysis acts as an underpinning backbone in drug development. Employing sophisticated measurement techniques, it furnishes critical data throughout the drug development journey. Starting from the initial sifting of candidate compounds, it helps in gauging their viability. Then, as drugs progress to the clinical stage, it enables personalized monitoring of drug use, ensuring optimal therapeutic outcomes. This process-from bench to bedside-is seamlessly supported by DMPK bioanalysis, whose precision-driven data is the linchpin for informed decision-making in drug development.
Discovery phase of drug development
When drug development is still in the blueprint stage of molecular design, DMPK bioanalysis has taken on the task of initial screening of drugability. In high-throughput screening scenarios, analytical technology needs to complete rapid quantification at the nanomolar concentration level to meet the evaluation needs of hundreds to thousands of candidate compounds per day. Ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) shows unique advantages at this stage. Through a 1.7μm filler column and an optimized mobile phase system, the analysis time of a single sample can be compressed to less than 90 seconds. When an innovative pharmaceutical company screened a library of 5,000 compounds, it used this technology to shorten the ADME screening cycle from 4 weeks of traditional methods to 3 days. This efficiency improvement is not only reflected in speed, but also in the multiplicity of analytical methods-parallel reaction monitoring (PRM) technology can set up multiple ion channels at the mass spectrometer end at the same time, and synchronously monitor the concentration changes of the parent drug and its characteristic metabolites, providing a direct basis for structural optimization for the development of drugs such as P38 MAPK inhibitors.
Bioanalysis in in vitro ADME experiments faces special challenges. Plasma protein binding rate detection often leads to false positive results due to adsorption on the well plate surface. A certain antiviral compound showed an 85% binding rate in a 96-well plate test, while the actual value after solid phase extraction (SPE) pretreatment was only 62%. To solve this kind of problem, dynamic dialysis has gradually replaced traditional equilibrium dialysis. By simulating the concentration difference on both sides of the membrane in the blood flow environment in the body, the correlation between the test data and the clinic has been improved by 40%. In cell uptake experiments, the combination of fluorescent labeling and flow cytometry can accurately quantify the efficiency of antibody drugs entering tumor cells. This single-cell level analysis provides key parameters for the linker design of antibody-drug conjugates (ADCs).
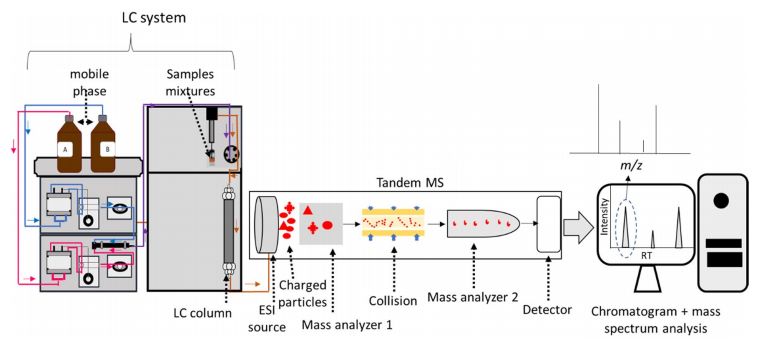 Fig. 1 Liquid chromatography–tandem mass spectrometry (LC–MS/MS) principles.1,4
Fig. 1 Liquid chromatography–tandem mass spectrometry (LC–MS/MS) principles.1,4
Service you may interested in
Preclinical stage of drug development
Entering preclinical research, the core task of DMPK bioanalysis shifts to crossing the barriers of species differences. In non-human primate experiments, α-2 macroglobulin in cynomolgus monkey plasma often forms complexes with therapeutic antibodies, resulting in ELISA test values that are 35% higher. However, the pretreatment step of Protein A affinity chromatography can effectively remove interfering proteins and control the test deviation within 5%. Species differences in metabolites are even more difficult. A statin mainly produces glucuronic acid conjugates in rats, while sulfated products are mainly produced in monkeys. The metabolite fingerprint library established by high-resolution mass spectrometry (HRMS) can systematically analyze such differences and provide a basis for the safety assessment of metabolites in toxicological studies.
Tissue distribution analysis achieves a leap from "concentration mean" to "spatial map" at this stage. Matrix-assisted laser desorption ionization mass spectrometry (MALDI-IMS) technology can draw a heat map of drug distribution with a resolution of 50μm. In the study of PD-1 inhibitors, this technology revealed that the concentration of drugs in tumor necrosis areas was 60% lower than that in proliferation areas. This discovery directly promoted the design of combined immunotherapy and radiotherapy. For oral solid preparations, bioanalysis also needs to focus on the first-pass metabolism of the gastrointestinal tract. Microdialysis technology is used to continuously collect rat portal vein blood samples, which can monitor the first-pass elimination rate of drugs after intestinal absorption in real time, providing dynamic data for dosage form optimization.
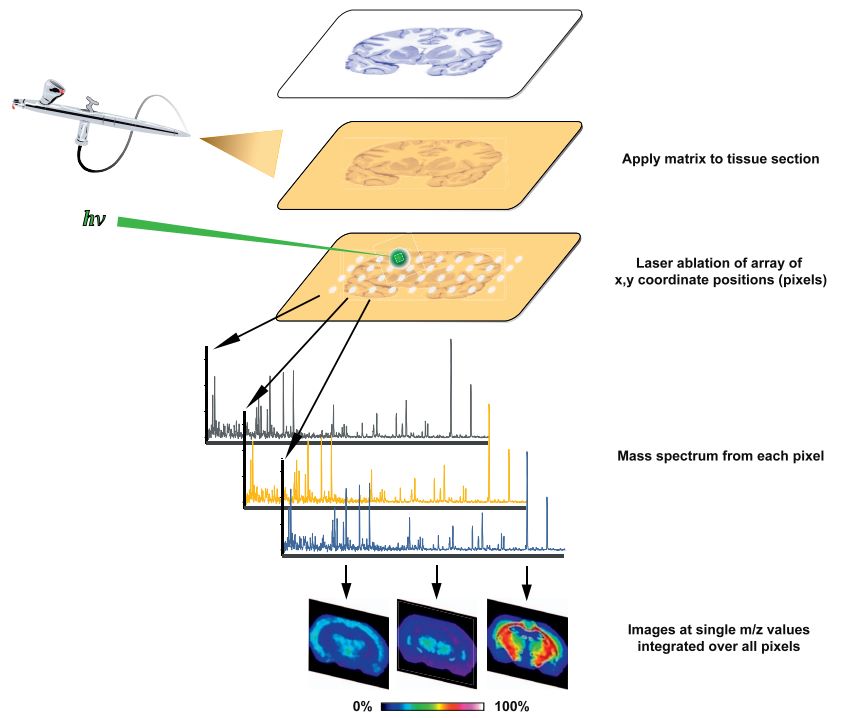 Fig.2 MALDI IMS process overview.2
Fig.2 MALDI IMS process overview.2
Clinical stage of drug development
In clinical trials and clinical applications, DMPK bioanalysis has been transformed into a core tool for therapeutic drug monitoring (TDM). Taking tacrolimus in the field of organ transplantation as an example, its therapeutic window is narrow (effective concentration 5-15 ng/mL). Insufficient concentration triggers rejection, while excessive concentration leads to nephrotoxicity. Bioanalysis has established a standardized monitoring system here: peripheral blood is collected every 12 hours within 72 hours after surgery, and a sample pretreatment process combining protein precipitation and solid phase extraction is used, combined with the multiple reaction monitoring (MRM) mode of triple quadrupole mass spectrometry to control the batch precision at RSD <5%. This precise measurement supports the dynamic adjustment of the dosing regimen. A transplant center has reduced the incidence of acute rejection from 28% to 9% one year after surgery by guiding medication through TDM.
Tumor immunotherapy promotes the development of bioanalysis towards multi-index co-detection. In the Phase III clinical trial of PD-1 antibody, researchers need to complete drug concentration detection and quantification of 12 cytokines (such as IL-6, IFN-γ) in 200μL plasma samples. Multiple ligand binding analysis (LBA) technology achieves this goal through microfluidic chips. Data analysis shows that the drug concentration is significantly positively correlated with the decrease in IL-6 (r=0.78), providing biomarker association evidence for drug efficacy prediction. For gene therapy drugs, bioanalysis also needs to face special challenges-the titer detection of adeno-associated virus (AAV) vectors uses a combination of qPCR and ELISA to ensure the accuracy of vector dosage through cross-validation of capsid protein quantification and genome copy number.
Technological evolution
Technological innovations in DMPK bioanalysis are driving its penetration from back-end testing to front-end decision-making. Some fully automated analysis platforms can complete plasma protein precipitation of 96 samples within 8 minutes and automatically generate data files that comply with FDA electronic record specifications, reducing manual operation errors by more than 80%. The AI-driven virtual mass spectrometry prediction model is based on the Transformer architecture. The mass spectrometry fragment ions can be predicted by inputting the compound structure with an accuracy rate of 91%. A multinational pharmaceutical company used this model to identify 3 potentially toxic metabolites in advance during the candidate compound optimization stage, avoiding resource waste in later research and development.
The introduction of blockchain technology has brought a revolutionary way of storing bioanalysis data. By storing LC-MS original maps, sample processing records, etc. on the chain through the Hyperledger platform, the whole process of data tampering can be traced. After a CRO adopted this technology, the review cycle of clinical application materials was shortened by 25%. Portable mass spectrometry equipment such as real-time direct analysis mass spectrometry (DART-MS) has broken through the limitations of laboratory scenarios. In brain tumor resection, surgeons can use DART-MS to scan the resected tissue on the spot and obtain the concentration distribution of the chemotherapy drug temozolomide within 10 minutes to guide the precise adjustment of the tumor resection range.
From high-throughput screening in the discovery stage to individualized monitoring in clinical applications, DMPK bioanalysis has always been centered on measurement science, building a line of defense for data quality at every key node in drug development. With the integration of new technologies such as single-cell analysis and spatial transcriptomics with traditional bioanalysis, this field is evolving from "drug concentration determination" to "in vivo behavior decoding", becoming a decisive scientific link connecting molecular design and clinical efficacy. In the future, when AI algorithms are able to directly optimize dosing regimens based on bioanalysis data, this technology will truly achieve the leap from "measurement" to "prediction", providing indispensable quantitative support for precision medicine.
Industry Challenges and Standard Construction
Despite the continuous technological innovation, DMPK bioanalysis still faces multiple challenges: detection of low-abundance markers in biological samples (such as pM-level Alzheimer's disease-related peptides in cerebrospinal fluid), interference removal in complex matrices (such as hemolysis and lipemia samples in blood), and standardized integration of multiple batches of data. To this end, the industry is promoting the construction of a standardized system: the "Biological Analytical Method Validation Guide" issued by the US FDA requires that the LLOQ must meet the drug concentration test of 3-5 half-lives, while the Chinese NMPA emphasizes the "fit-for-purpose" principle of bioanalytical methods and adjusts the validation standards according to the research and development stage.
In the context of global research and development of innovative drugs, cross-laboratory data comparability has become the key. In an international multicenter clinical trial, 12 laboratories used a unified tacrolimus detection method (UPLC-MS/MS) and calibrated with matrix-matched standards to achieve batch-to-batch precision RSD <8%, providing reliable TDM data support for multinational pharmaceutical companies. In addition, blockchain technology is being tried to be applied to the traceability of biological analysis data, recording the entire process of sample pre-processing, instrument parameters, and data analysis through distributed ledgers to ensure data integrity and traceability.
From nanomolar molecular screening to spatial imaging at the single-cell level, from species difference analysis in animal models to individualized monitoring in human clinical settings, DMPK bioanalysis has always been centered on "precise measurement" and plays multiple roles in the drug development chain: "molecular balance," "species translator," and "clinical calibrator." With mass spectrometry resolution exceeding 200,000 FWHM and single-cell detection throughput increasing to millions per day, this discipline is accelerating the transformation of drug development from "experience-driven" to "data-driven." In the future, when AI algorithms are deeply integrated with microfluidic chips, DMPK bioanalysis may achieve a leap from "post-measurement" to "pre-prediction," providing more accurate technical support for conquering major diseases such as tumors and neurodegenerative diseases.
If you want to learn more about the DMPK, please refer to:
References
- Dewi, Kifayati Rosiyanti, et al. "Advances and key considerations of liquid chromatography–mass spectrometry for porcine authentication in halal analysis." Journal of Analytical Science and Technology 14.1 (2023): 13. https://doi.org/10.1186/s40543-023-00376-3
- Stark, David T., et al. "Optic nerve regeneration after crush remodels the injury site: molecular insights from imaging mass spectrometry." Investigative ophthalmology & visual science 59.1 (2018): 212-222. https://doi.org/10.1167/iovs.17-22509
- Higgins, Luke, Henry Gerdes, and Pedro R. Cutillas. "Principles of phosphoproteomics and applications in cancer research." Biochemical Journal 480.6 (2023): 403-420. https://doi.org/10.1042/BCJ20220220
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
