How to Create Humanized Mice: A Step-by-Step Guide for Researchers
Introduction: The Core Value of Humanized Mice
Humanized mouse models represent a groundbreaking category of living chimeric organisms engineered to incorporate functional human genes, cells, or tissues. These models bridge the significant biological gap between preclinical animal studies and human physiology, addressing a critical challenge in biomedical research: the 89% failure rate of drug trials that succeed in conventional animal models but fail in humans. By recapitulating human-specific biological processes, humanized mice enable researchers to conduct preclinical studies with unprecedented translational relevance.
The scientific significance of humanized mice lies in their ability to model human-specific diseases and physiological responses. Key applications span three major domains:
- Immunotherapy development: Evaluating the efficacy of novel therapies like anti-PD-1 antibodies in a human immune context.
- Infectious disease modeling: Studying pathogens such as HIV and HCV, which often fail to replicate in conventional mouse models.
- Toxicity and efficacy studies: Assessing human-specific drug responses and adverse effects that are unobservable in non-humanized animals.
Universal Construction Workflow for Humanized Mice
1. Objective Design
The first step in constructing a humanized mouse model is defining the research objective, which dictates the methodology:
- Immune system studies typically require cell transplantation to reconstitute human immune components.
- Target validation for gene therapy or drug discovery often necessitates genetic engineering to introduce human genes.
2. Recipient Selection
Choosing the right host mouse strain is critical for successful human cell or tissue engraftment:
- Immunodeficient strains: NSG (NOD.scid.IL2Rγnull) mice offer the highest engraftment efficiency, outperforming NOG and NOD/SCID strains by up to sixfold due to their severe combined immunodeficiency.
- Genetic engineering hosts: C57BL/6 zygotes or embryonic stem (ES) cells are commonly used for gene editing due to their genetic stability and accessibility.
3. Core Procedure Execution
Cell Transplantation
- Preconditioning: Recipient mice undergo sublethal irradiation to suppress the native immune system, creating a niche for human cells.
- Cell delivery: Human cells are introduced via intrahepatic injection (for neonatal mice) or intravenous infusion, depending on the cell type and study goal.
Genetic Engineering
- CRISPR/Cas9 technology: Commonly used for precise gene editing, involving microinjection of sgRNA and Cas9 into zygotes or electroporation of ES cells.
- Homology-directed repair: Utilizes single-stranded oligodeoxynucleotides (ssODNs) to facilitate human gene integration.
4. Embryo Transfer and Post-Operative Care
- Embryo transfer: Edited zygotes or transplanted embryos (at E2.5) are implanted into pseudopregnant female mice.
- Post-surgical care: Mice require strict thermoregulation (37°C) until fully recovered to ensure optimal survival and development.
5. Validation Metrics
- Cell-based models: Flow cytometry confirms human cell engraftment (e.g., hCD45+ cell levels >15%).
- Genetic models: Sanger sequencing and Western blotting verify gene editing efficiency and protein expression.
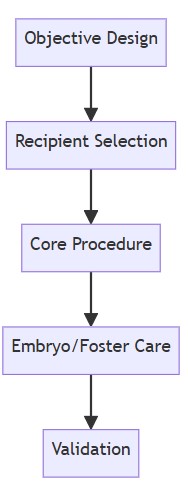 Fig.1 A brief flow chart for the construction of humanized mice.
Fig.1 A brief flow chart for the construction of humanized mice.
Service you may interested in
Classification of Humanized Mouse Construction Methods
Cell Transplantation-Based Models
1. Hu-PBL-SCID (Peripheral Blood Mononuclear Cell Transplantation)
- Principle: Rapid reconstitution of human T cells via tail vein injection of PBMCs.
- Technical route: Inject 2-10×10⁶ PBMCs into SCID mice, with immune reconstitution occurring within 3-4 weeks.
-
Advantages/limitations:
✅ Fast setup (3-4 weeks) and simple protocol.
❌ High risk of graft-versus-host disease (GVHD) and short viability (<8 weeks). - Applications: Acute immune response studies, such as antibody pharmacodynamics.
2. Hu-SRC-SCID (CD34+ Hematopoietic Stem Cell Transplantation)
- Principle: Long-term multi-lineage immune reconstruction using CD34+ HSCs.
-
Technical route:
- Sublethal irradiation (1Gy) of NSG mice to prepare the recipient.
- Intrahepatic injection of high-purity (>90%) CD34+ cells from umbilical cord or fetal liver into neonatal mice.
-
Advantages/limitations:
✅ Sustained engraftment for over 6 months and lymphoid cell differentiation.
❌ Impaired myeloid cell development and lack of thymic education. - Applications: Hematopoietic disorder research and chronic viral infection modeling.
3. BLT Model (Bone Marrow-Liver-Thymus Co-Transplantation)
- Principle: Creates a functional human immune microenvironment through fetal tissue transplantation.
-
Technical route:
- Subrenal capsule implantation of 1.6mm³ fetal liver and thymus fragments.
- Intravenous boost of CD34+ HSCs to enhance engraftment.
-
Advantages/limitations:
✅ Functional T-cell education and high engraftment levels.
❌ Requires complex microsurgery and has a high GVHD incidence. - Applications: HIV latency studies and tumor microenvironment modeling.
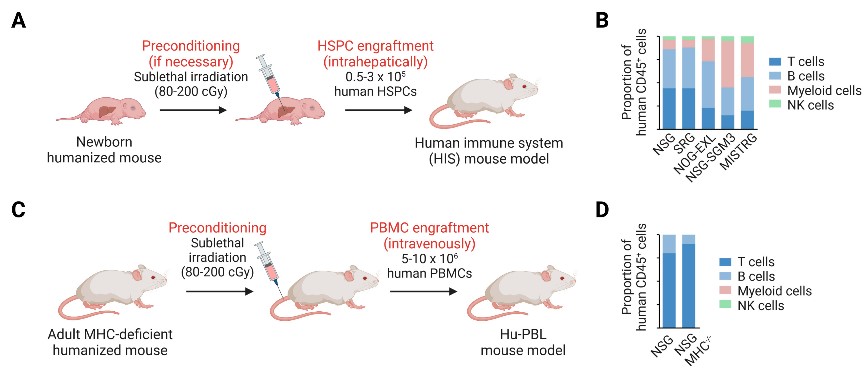 Fig. 2 Generation of humanized immune system mouse models.1,2
Fig. 2 Generation of humanized immune system mouse models.1,2
Genetic Engineering-Based Models
1. Partial Gene Replacement (e.g., Antibody Humanization)
- Principle: Swaps murine gene domains with human equivalents to produce human-compatible antibodies.
-
Technical route:
- CRISPR/Cas9-mediated recombination with ssODNs.
- Homology arms (>1kb) flanking humanized sequences to ensure precise integration.
-
Advantages/limitations:
✅ Enables production of high-affinity human antibodies and species-compatible immune responses.
❌ Limited to targeted pathways and specific gene modifications. - Applications: Therapeutic antibody screening, such as CD3-specific immunotherapies.
2. Whole Gene Network Humanization
- Principle: Reconstructs cross-species signaling pathways to mimic human physiological responses.
-
Technical route:
- TurboKnockout-Pro (or similar high-capacity editing tools) for inserting 200kb+ gene clusters.
- ES cell targeting followed by blastocyst injection for germline transmission.
-
Advantages/limitations:
✅ Resolves cytokine and receptor incompatibility issues between species.
❌ Lengthy development time (>8 months) and potential off-target effects. - Applications: Natural killer (NK) cell therapy evaluation and inflammatory disease modeling.
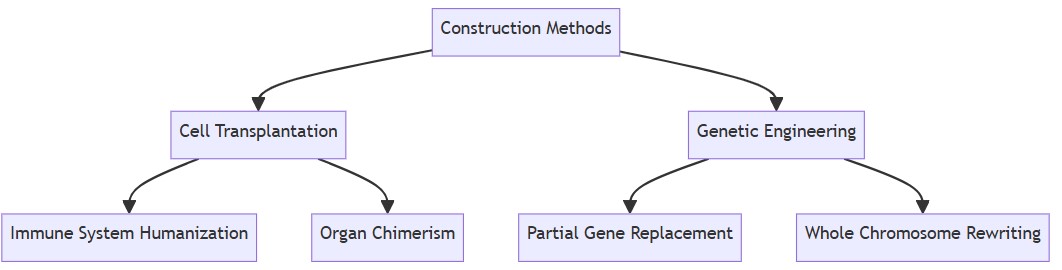 Fig.3 Classification of methods for constructing humanized mouse models.
Fig.3 Classification of methods for constructing humanized mouse models.
Technology Comparison and Optimization
| Method | Key Advantage | Primary Limitation | Ideal Application |
|---|---|---|---|
| PBMC Transplant | Rapid engraftment (3-4 weeks) | Severe GVHD | Acute immune response studies |
| CD34+ HSC Transplant | Multi-lineage immune differentiation | Myeloid cell deficiency | Hematopoietic disorders and chronic infections |
| BLT Model | Functional T-cell education | Surgical complexity | HIV research and T-cell immunity studies |
| Partial Gene Editing | Targeted human antibody production | Pathway-specific limitations | Therapeutic antibody screening |
| Whole Network Editing | Cross-species physiological compatibility | Lengthy development time | Cytokine therapy and immune disorder modeling |
Recent Advances and Case Studies
1. Dual-Humanized Innovations
- Liver-immune chimeras: Models combining human liver cells and immune components have been used to study anti-CD95-induced liver injury, coupled with human bone marrow mesenchymal stem cell (hBMSC) transplantation for regenerative medicine research.
2. Oncology Applications
- Patient-derived xenograft (PDX) models: When combined with immune reconstitution, PDX models enable more accurate evaluation of cancer therapies in a human-like tumor microenvironment.
3. CRISPR-Cas9 Breakthroughs
- Single-step gene network humanization: Techniques allowing simultaneous editing of cytokine networks (e.g., IL-15/IL-15R) have enhanced NK cell therapy development by improving human immune cell functionality in mice.
Humanized mouse models have revolutionized preclinical research by bridging the translational gap between animal studies and human biology. From cell transplantation approaches that reconstitute human immune systems to genetic engineering techniques that mimic human gene networks, these models continue to evolve, driving breakthroughs in immunotherapy, infectious disease research, and personalized medicine. As CRISPR technology and transplantation methods advance, humanized mice will remain indispensable tools for accelerating drug development and unraveling the complexities of human disease.
Unlock Your Research Potential with Creative Biolabs' Humanized Mouse Models
At Creative Biolabs, we pride ourselves on our extensive experience in constructing humanized mouse models. We've successfully partnered with numerous pharmaceutical companies and research institutions, empowering their studies with our expertise.
If you have specific experimental requirements for humanized mouse models, don't hesitate to contact us! Our team is ready to provide you with a detailed and tailored construction plan to help you achieve your research goals.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Chen, Anna, Ines Neuwirth, and Dietmar Herndler-Brandstetter. "Modeling the tumor microenvironment and cancer immunotherapy in next-generation humanized mice." Cancers 15.11 (2023): 2989. https://doi.org/10.3390/cancers15112989
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
