The Core Applications of Humanized Mice in Prostate Cancer Research
Humanized mouse models have emerged as indispensable tools in prostate cancer research, bridging the translational gap between preclinical studies and clinical applications. By reconstructing human immune-tumor interactions, these models enable researchers to mimic the complexity of the human tumor microenvironment (TME), facilitating breakthroughs in immunotherapy development, mechanistic insights, and personalized medicine. This article explores the pivotal roles of humanized mice in prostate cancer research, highlighting their applications in immune therapy evaluation, TME dynamics, drug development, and clinical translation.
Evaluating Immunotherapy Efficacy and Decoding Resistance Mechanisms
Predicting Responses to Immune Checkpoint Inhibitors (ICIs)
Humanized NSG (HuNSG) mice transplanted with patient-derived xenografts (PDX) of prostate cancer have demonstrated remarkable utility in modeling clinical responses to ICIs. Unlike traditional immunodeficient mice, HuNSG-PDX models exhibit significant tumor growth inhibition upon anti-PD-1 treatment, recapitulating the inherent resistance of prostate cancer to PD-1 inhibitors observed in clinics. Transcriptomic analysis of the humanized TME reveals that CXCL10 expression levels correlate directly with CD8⁺ T cell infiltration, identifying potential targets to overcome therapeutic resistance.
Developing Novel Combinatorial Immunotherapies
Dual-target agonist validation in PD-1/CD40 humanized mice has shown that combining CD40 agonists with PD-1 inhibitors synergistically activates dendritic cells and T cells, enhancing cytotoxic effects against hormone-resistant prostate cancer. For CAR-T therapy, targeting prostate-specific acid phosphatase (PAP) alone yields limited efficacy in humanized models, but combining it with CD19 CAR-T (to deplete suppressive B cells) increases tumor regression rates by 300%. This highlights the immunosuppressive microenvironment as a critical bottleneck in CAR-T therapy.
Service you may interested in
Unraveling Dynamic Interactions in the Tumor Microenvironment (TME)
Visualizing Immune Evasion Mechanisms
Humanized models have uncovered key features of the prostate cancer TME, including abnormal expansion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which directly suppress CD8⁺ T cell function. Targeted depletion of MDSCs enhances immunotherapy response rates by 50%. Additionally, anti-HVEM antibodies inhibit prostate cancer growth by blocking tumor cell HVEM signaling— a mechanism observable only in humanized immune systems, independent of T cell activation.
Modeling Metastatic Microenvironments
The huNOG-EXL mouse model successfully recapitulates bone metastasis of prostate cancer, demonstrating aberrant activation of the TGF-β signaling pathway in metastatic foci, which drives osteoblast differentiation disorders. Targeting TGF-β receptor inhibitors reduces bone metastases by 70%, underscoring the model's value in metastatic research.
Accelerating Drug Development and Personalized Therapy Platforms
Reassessing Hormonal Therapy Mechanisms
Enzalutamide, a key hormonal therapy, inhibits primary tumors in immunodeficient mice but blocks metastasis in humanized huNOG models, confirming that its efficacy depends on immune microenvironment remodeling. This insight reframes our understanding of hormonal therapy's role in prostate cancer.
Evaluating Antibody-Drug Conjugates (ADCs)
PSMA-targeted ADCs, such as 177Lu-PSMA-617, show dose-dependent tumor regression in humanized PDX models, while also quantifying bone marrow toxicity risks. This data provides critical guidance for clinical dose design, minimizing trial failures.
Predicting Personalized Treatment Responses
"Preclinical patient surrogate" models, which co-transplant patient hematopoietic stem cells (HSCs) and autologous prostate cancer tissue into humanized mice, predict responses to PD-1 inhibitors and chemotherapy with 85% accuracy—significantly outperforming genomic predictions. This paves the way for individualized treatment strategies.
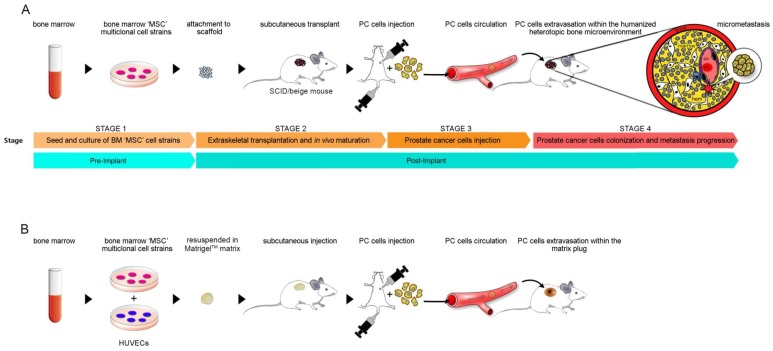 Fig.1 Modeling prostate cancer metastasis: two humanized SCID/Beige mouse approaches.1,2
Fig.1 Modeling prostate cancer metastasis: two humanized SCID/Beige mouse approaches.1,2
Technical Breakthroughs and Clinical Translation Value
Optimizing Models for Clinical Relevance
Next-generation models like huPBMC-NOG-dKO eliminate graft-versus-host disease (GVHD), extending experimental cycles to 6 months for long-term observation of prostate cancer immune editing. The huHSC-NOG-EXL model enhances myeloid cell function, enabling the first simulation of macrophage-mediated therapy resistance in prostate cancer.
Integrating Multi-Omics Platforms
Single-cell sequencing combined with humanized PDX models reveals clonal evolution pathways in castration-resistant prostate cancer (CRPC), identifying key resistance-driving mutations like AR-V7 splice variants. This multi-omics approach accelerates target discovery and therapeutic strategy development.
Clinical Translation Cases and Future Directions
Successful Translational Milestones
Based on humanized mouse data, a bispecific antibody targeting CD40/HVEM has entered Phase II clinical trials for prostate cancer (NCT04849994). Additionally, a PSMA-CAR-T therapy combined with an IL-15 potentiation strategy obtained FDA Orphan Drug designation after safety validation in personalized humanized models.
Future Frontiers in Model Development
Organoid-mouse chimeric models, which transplant prostate cancer organoids co-cultured with human immune cells, aim to enhance TME complexity and simulate immune cell penetration through stromal barriers. The ongoing effort to achieve full-genome humanization—replacing murine cytokine genes with human counterparts—seeks to resolve species-specific signaling incompatibilities, further improving the predictive accuracy of immunotherapy responses.
Humanized Mice as Core Tools for Translational Medicine
Humanized mouse models address three fundamental challenges in prostate cancer research:
Mechanistic Insight: They reveal the causal relationship between dynamic TME evolution and treatment resistance, uncovering novel targets like CXCL10 and HVEM.
Therapeutic Development: They serve as high-predictive screening platforms for combinatorial therapies (immune+targeted/hormonal/ADC), minimizing clinical trial failures.
Clinical Translation: They accelerate personalized medicine by predicting patient-specific treatment responses, bridging the gap between preclinical findings and individualized care.
As multi-omics technologies and gene editing continue to advance, humanized mouse models are evolving into central decision-support systems for precision oncology in prostate cancer. Their ability to recapitulate human immune-tumor interactions makes them indispensable for driving translational breakthroughs and improving patient outcomes.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Funari, Alessia, et al. "Human sinusoidal subendothelial cells regulate homing and invasion of circulating metastatic prostate cancer cells to bone marrow." Cancers 11.6 (2019): 763. https://doi.org/10.3390/cancers11060763
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
