Humanized Mice in Breast Cancer Research: Modeling Tumor Progression & Treatment
The Critical Need for Humanized Models in Breast Cancer Research
Breast cancer research faces a significant translational gap, where preclinical findings often fail to translate to clinical success due to species-specific differences between murine and human biological systems. Traditional xenograft models, which rely on immunocompromised mice, lack functional human immune components and tumor microenvironments, limiting their ability to recapitulate human tumor-immune interactions. Humanized mice, however, offer a revolutionary approach by integrating human immune cells, tissues, or genetic elements, enabling more physiologically relevant modeling of tumor progression, metastasis, and therapeutic responses.
This article explores the role of humanized mouse models in breast cancer research, focusing on their applications in tumor progression modeling, immunotherapy evaluation, and drug screening. By bridging the gap between preclinical and clinical studies, these models have become indispensable tools for accelerating breast cancer therapy development.
Types of Humanized Mouse Models in Breast Cancer Research
Immune System Humanization Approaches
CD34+ Hematopoietic Stem Cell (HSC) Models
CD34+ HSC transplantation into immunodeficient mice (e.g., NSG or NOG mice) enables long-term engraftment of human immune cells, including T cells, B cells, and myeloid cells. This model is ideal for chronic immunotherapy studies, such as evaluating the efficacy of CAR-T cell therapies over extended periods. The slow immune reconstitution (4–6 months) allows for the development of a mature human immune system, making it suitable for studying adaptive immune responses.
Peripheral Blood Mononuclear Cell (PBMC) Models
PBMC models involve the transplantation of human peripheral blood cells, providing a rapid (2–4 week) reconstitution of T and NK cells. This approach is well-suited for short-term studies of cell-mediated immunity, such as assessing NK cell-mediated tumor lysis. However, it carries risks of graft-versus-host disease (GvHD), limiting its long-term applicability.
BLT (Bone Marrow-Liver-Thymus) Models
BLT models represent the most comprehensive human immune system reconstitution, achieved by co-transplanting human fetal liver and thymus tissue with HSCs. This method supports the development of functional lymphoid organs and a diverse immune repertoire. However, ethical concerns regarding fetal tissue use restrict its widespread adoption.
Tumor Engraftment Strategies
Cell Line-Derived Xenografts (CDX)
CDX models use established breast cancer cell lines (e.g., MCF-7, MDA-MB-231) for tumor transplantation, offering standardized and reproducible tumor growth kinetics. These models are valuable for initial drug screening but may lack the genetic heterogeneity of primary tumors.
Patient-Derived Xenografts (PDX)
PDX models involve transplanting patient tumor tissue into immunodeficient mice, preserving tumor heterogeneity and microenvironmental components. PDX models have shown particularly high success rates in triple-negative breast cancer (TNBC), with engraftment efficiencies reaching up to 85%. They are critical for studying tumor evolution and personalized medicine.
Humanized Microenvironment Models
To mimic metastatic niches, researchers co-transplant human fibroblasts, endothelial cells, or bone tissue fragments. For example, human bone implants enable modeling of osteolytic bone metastasis, a common complication in advanced breast cancer.
Transgenic Humanized Models
Genetically engineered mice with humanized immune checkpoints (e.g., PD-1, PD-L1, CTLA-4 knock-ins) or cytokine profiles (e.g., MISTRG, NSG-SGM3 mice for myeloid cell studies) enhance the physiological relevance of models. These mice allow for more accurate testing of immune checkpoint inhibitors and cytokine-based therapies.
Service you may interested in
Applications in Breast Cancer Progression and Therapy
Modeling Tumor Progression and Metastasis
Humanized mice facilitate the study of breast cancer metastasis to specific organs. For instance, bone metastasis models using human bone fragments enable researchers to investigate osteoclast-mediated tumor growth and test anti-resorptive therapies. Co-transplantation of human cancer-associated fibroblasts (CAFs) enhances orthotopic tumor engraftment, highlighting the role of stromal cells in tumor progression.
Immunotherapy Evaluation
Immune Checkpoint Inhibitors
In TNBC PDX models, anti-PD-1 antibodies have demonstrated efficacy through CD8+ T-cell-dependent tumor regression, recapitulating clinical responses. These models help optimize combination strategies, such as pairing PD-1 blockade with 4-1BB agonists to enhance T-cell activation.
CAR-T Cell Therapy
Humanized mice allow in vivo assessment of CAR-T cell safety and efficacy in breast cancer. Preclinical studies using these models have explored CARs targeting antigens like HER2, providing insights into tumor-specific killing and potential off-tumor toxicities.
Combination Therapies
Humanized models excel at evaluating synergistic effects between immunotherapies and chemotherapy/radiation. For example, they have shown enhanced tumor regression when combining anti-PD-L1 with DNA-damaging agents, supporting clinical trial design.
Drug Screening and Personalized Medicine
Hu-PDX platforms (humanized patient-derived xenografts) integrate human immune cells and patient tumors, enabling prediction of individual drug responses. Additionally, mice engineered to express human cytochrome P450 enzymes facilitate pharmacokinetic studies, improving translation of drug metabolism data to humans.
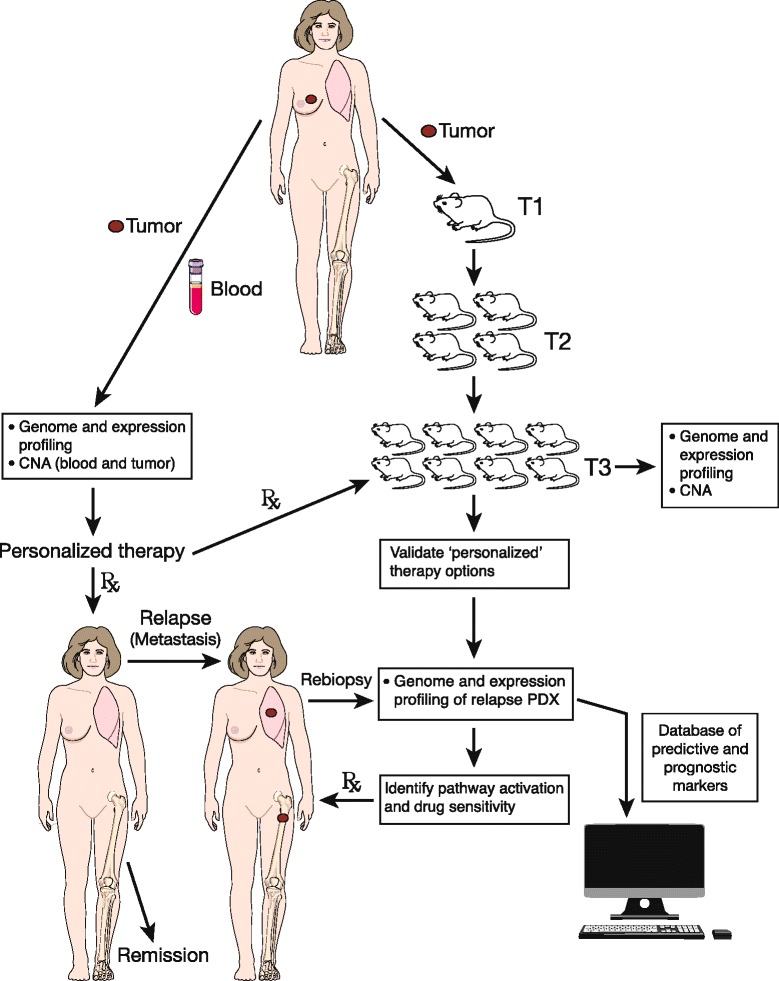 Fig.1 Combining mouse PDX data with breast cancer patient treatment.1,2
Fig.1 Combining mouse PDX data with breast cancer patient treatment.1,2
Key Advantages Over Traditional Xenograft Models
| Feature | Xenograft Models | Humanized Mice |
|---|---|---|
| Immune System | Mouse (absent/compromised) | Functional human immune cells |
| Tumor Microenvironment | Mouse stroma dominates | Human stroma integration |
| Immunotherapy Testing | Limited relevance | Clinically predictive responses |
| Metastasis Modeling | Low incidence | Enhanced with humanized tissues |
Humanized mice offer three core advantages:
- Biological Relevance: By recapitulating human immune-tumor interactions, they provide more accurate preclinical data.
- Translational Value: Responses to immunotherapies in humanized models often mirror clinical outcomes, reducing trial failures.
- Mechanistic Insight: They enable detailed studies of tumor-immune crosstalk, guiding the development of targeted therapies.
Challenges and Limitations
Technical Constraints
- High costs (>$5,000 per model) and lengthy generation times (4–6 months) limit large-scale use.
- Variable engraftment efficiency: ER+ breast tumors show only ~9% engraftment rates in PDX models, compared to TNBC's 85%.
Immune System Deficiencies
- Innate immune cells (neutrophils, dendritic cells) are often underrepresented, affecting innate immunity studies.
- Incomplete lymphoid organ development (e.g., lack of functional tonsils or adenoids) may impact B-cell responses.
Physiological Discrepancies
- Telomerase activity differences between human and mouse cells can affect tumor cell immortalization.
- Divergent cytokine signaling (e.g., human IL-15 vs. mouse IL-15) may alter immune cell function.
Ethical Considerations
BLT models rely on fetal tissue, raising ethical concerns. Alternative approaches using induced pluripotent stem cells (iPSCs) are being explored to address this issue.
Future Directions and Innovations
Next-Generation Autograft Systems
Models using patient-matched HSCs and tumors (autograft systems) aim to eliminate immune rejection and enhance personalization. This approach could revolutionize preclinical testing for individual patients.
Improved Immune Reconstitution
- HLA-engineered mice for human antigen presentation, enabling studies of T-cell receptor (TCR) therapies.
- FLT3L/IL-6 cytokine supplementation to promote myeloid cell development, addressing innate immune deficiencies.
Multi-Organ Humanization
Co-engraftment of human liver, lymph nodes, and other organs will enable modeling of systemic immune responses and drug metabolism, improving translational accuracy.
Standardization and Repositories
Establishing PDX model repositories with standardized characterization (genomic, transcriptomic data) will accelerate collaborative research and reduce redundancy.
Bridging Preclinical Research and Clinical Application
Humanized mouse models have emerged as indispensable tools in breast cancer research, addressing the translational gap by providing physiologically relevant systems for tumor modeling and therapeutic testing. Their ability to recapitulate human immune-tumor interactions has significantly advanced immunotherapy development, particularly for checkpoint inhibitors and CAR-T therapies.
To maximize their utility, researchers must adopt best practices in model selection: CD34+ HSC models for long-term immunotherapy studies, PBMC models for rapid NK-cell assessments, and PDX models for personalized medicine. Moving forward, integrating humanized mice with technologies like optical imaging and single-cell sequencing will further enhance mechanistic insights.
As breast cancer research continues to prioritize precision medicine, humanized mice will play a pivotal role in de-risking clinical trials and accelerating the development of effective, patient-specific therapies. Their evolution toward more complex, multi-organ systems holds promise for transforming preclinical research and improving patient outcomes.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Whittle, James R., et al. "Patient-derived xenograft models of breast cancer and their predictive power." Breast cancer research 17 (2015): 1-13. https://doi.org/10.1186/s13058-015-0523-1
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
