Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
Introduction to In Vitro ADME Studies
In the realm of drug discovery, in vitro ADME (Absorption, Distribution, Metabolism, Excretion) studies represent a cornerstone of preclinical evaluation. These studies involve a battery of laboratory-based assays designed to characterize how potential drug candidates interact with biological systems at the cellular and molecular levels. The core objective is to predict key pharmacokinetic (PK) properties—how a drug is absorbed into the bloodstream, distributed to target tissues, metabolized into active or inactive byproducts, and excreted from the body.
By providing insights into these fundamental processes, in vitro ADME studies play a pivotal role in optimizing candidate drug design. They enable researchers to identify compounds with suboptimal ADME profiles early in the discovery pipeline, such as those prone to rapid metabolism or poor oral absorption, thereby reducing the likelihood of late-stage clinical failures. This proactive approach aligns with a model-driven strategy that aims to forecast PK behavior in animals and humans, minimizing development costs and accelerating the transition from lead identification to clinical trials.
Historical Evolution
The significance of in vitro ADME testing emerged from the lessons of pharmaceutical history. In the 1990s, drug development was plagued by high clinical failure rates, with 40-50% of candidates failing due to unanticipated ADME-related issues, such as severe drug-drug interactions or inadequate bioavailability. The turning point came in the early 2000s with the integration of systematic in vitro ADME screens into the drug discovery workflow.
This era witnessed two transformative advancements: the widespread adoption of human-derived biological reagents, such as liver microsomes and primary hepatocytes, which offered more physiologically relevant data than animal-based models, and the introduction of high-throughput screening (HTS) technologies. These innovations allowed researchers to profile hundreds of compounds per week, shifting the paradigm from reactive problem-solving to proactive property-based selection. Consequently, the failure rate attributed to ADME liabilities dropped significantly to around 10%, marking a pivotal shift toward more efficient drug development.
Key Components of ADME Assays
Absorption Studies
Understanding a drug's absorption potential is critical for oral drug candidates, as poor absorption directly impacts bioavailability. Three primary methodologies dominate this space:
- Caco-2/MDCK Cell Models: These epithelial cell lines, derived from human colorectal adenocarcinoma (Caco-2) or canine kidney (MDCK), form confluent monolayers that mimic the intestinal epithelium. By measuring transcellular permeation rates, these models assess both passive diffusion and active transport mechanisms, providing insights into intestinal absorption.
- Parallel Artificial Membrane Permeability Assay (PAMPA): A simplified in vitro system using synthetic lipid membranes, PAMPA quantifies passive trans-membrane diffusion, particularly useful for ranking compounds based on their lipophilicity-driven permeability.
- Solubility Testing: Differentiating between kinetic and thermodynamic solubility is essential. Kinetic solubility measures dissolution rates under non-equilibrium conditions, while thermodynamic solubility assesses equilibrium concentrations. Both parameters influence formulation design and ultimate bioavailability, as poorly soluble compounds often exhibit erratic absorption.
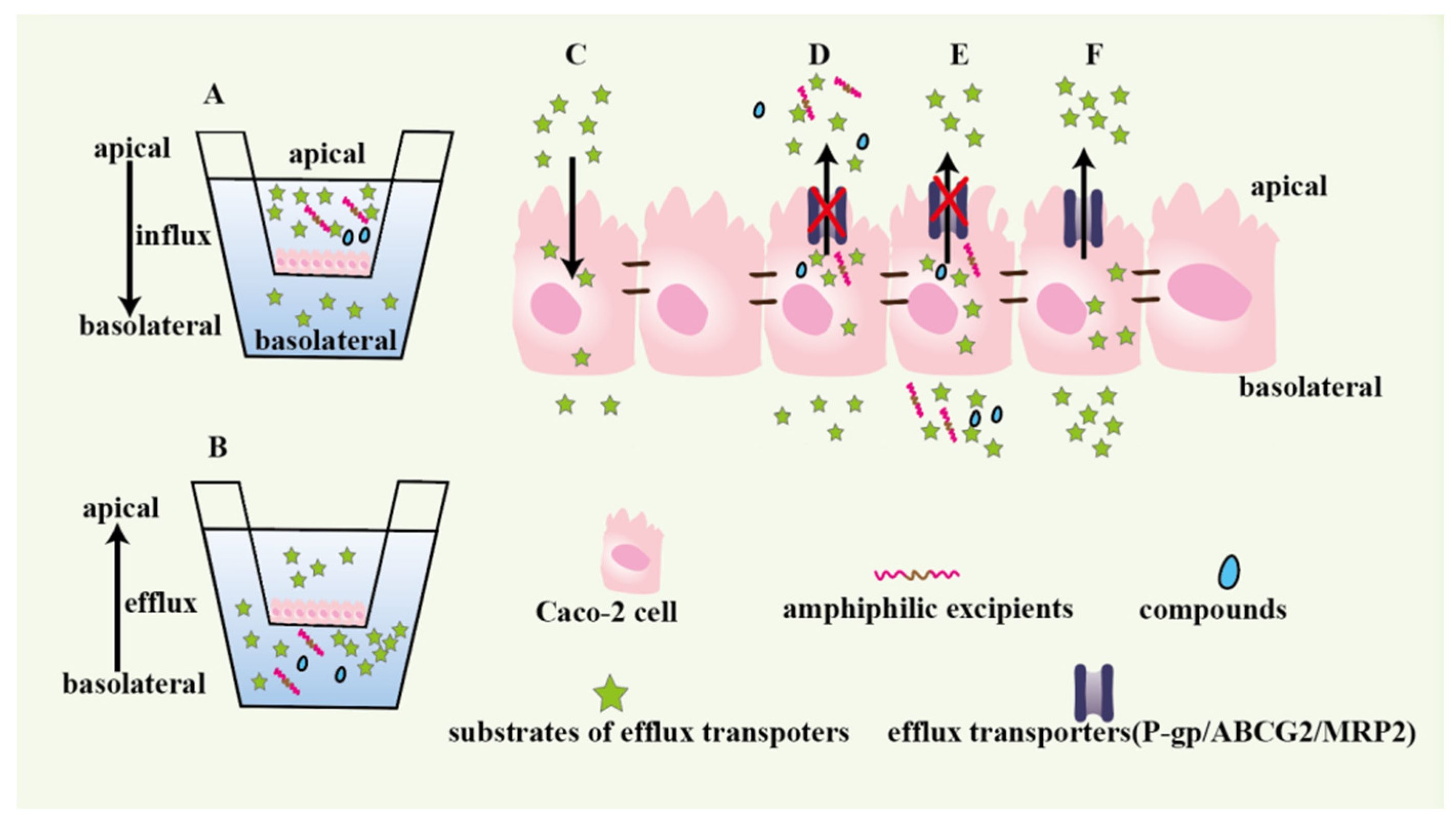 Fig.1 Caco-2 Model for Drug Absorption, Efflux, and Excipient Studies.1,2
Fig.1 Caco-2 Model for Drug Absorption, Efflux, and Excipient Studies.1,2
Distribution Profiling
Drug distribution within the body is governed by two key processes:
- Plasma Protein Binding Assays: Measuring the fraction of free (unbound) drug in plasma is critical, as only unbound drug can distribute to tissues or exert pharmacological effects. High binding rates (e.g., >90%) may limit tissue penetration and increase vulnerability to drug-drug interactions via protein binding displacement.
- Quantitative Tissue Distribution Studies: These studies quantify drug accumulation in various organs, providing data on target engagement and potential off-target toxicity. Techniques such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) enable sensitive quantification across tissue matrices, guiding dose optimization and risk assessment.
Metabolism Analysis
The liver is the primary site of drug metabolism, making hepatic assays central to ADME profiling:
- Liver Microsome Stability Tests: Incubating compounds with human liver microsomes (a source of cytochrome P450 enzymes) evaluates metabolic clearance rates. Rapid metabolism indicates high first-pass extraction, which may necessitate parenteral administration or structural modification to improve half-life.
- CYP450 Enzyme Inhibition/Induction Assays: Identifying potential interactions with cytochrome P450 isoforms (e.g., CYP3A4, CYP2D6) is critical to avoid adverse drug-drug interactions. Inhibition studies measure reductions in enzyme activity, while induction assays assess changes in gene expression, both of which are mandatory for regulatory submissions.
Service you may interested in
Excretion Balance
Characterizing drainage pathways ensures a comprehensive understanding of drug elimination:
- Excretion Pathway Analysis: Quantifying renal (urine) and biliary (feces) excretion rates helps determine the primary elimination route. For example, renal clearance is critical for water-soluble compounds, while biliary excretion dominates for larger molecules.
- Mass Balance Studies: By tracking the total recovery of radio-labeled drugs and their metabolites in excreta, these studies validate metabolic pathways and ensure mass closure (typically requiring ≥80% recovery), which is essential for regulatory compliance.
Advanced Techniques in ADME Studies
Metabolite Identification and Profiling
Modern analytical technologies have revolutionized metabolite characterization:
- High-Resolution Mass Spectrometry (HRMS): With precision mass measurements (ppm-level accuracy), HRMS enables definitive elemental composition determination and fragmentation pattern analysis, facilitating the identification of novel metabolites even at low abundances.
- Isotope Filtering and Defect Filtering: By utilizing stable isotope-labeled standards or analyzing mass defect signatures, these techniques distinguish true metabolites from background noise, enhancing the reliability of metabolic profiling.
- NMR-LC-MS/MS Hyphenation: Combining nuclear magnetic resonance (NMR) for structural confirmation with LC-MS/MS for quantitative tracking allows dynamic mapping of metabolic pathways, resolving complex transformation sequences.
Quantitative Tissue Distribution Studies
Data interpretation in tissue distribution analysis is enhanced by:
- Spatial Registration: Standardizing anatomical coordinates across tissue samples using reference atlases minimizes variability in regional drug concentration measurements, enabling accurate inter-sample comparisons.
- Time Registration: Aligning time-course data with biological rhythms (e.g., circadian cycles affecting organ physiology) ensures that kinetic profiles reflect true tissue dynamics, improving the predictive value for in vivo PK modeling.
Physiologically Based Pharmacokinetic (PBPK) Modeling
PBPK models integrate in vitro ADME data (e.g., solubility, permeability, metabolic rates) with physiological parameters (e.g., organ blood flow, tissue composition) to simulate drug distribution in humans. These mechanistic models predict critical PK parameters—such as volume of distribution, clearance, and half-life—allowing rational dose selection and identification of population-specific differences (e.g., age, disease states), thus optimizing clinical trial designs.
Impact of ADME Testing in Drug Discovery
Case Studies
- Case 1: Accelerated Lead Optimization: Implementing a high-throughput ADME screening cascade (processing over 300 compounds weekly) enabled a research team to prioritize candidates with balanced absorption, moderate plasma protein binding, and favorable metabolic stability. This approach reduced lead optimization cycles by 40%, significantly lowering R&D investments by eliminating non-viable candidates early.
- Case 2: Avoiding Late-Stage Failure: A compound with promising in vitro efficacy was flagged in liver microsome assays for extremely rapid metabolism (half-life <5 minutes). Further evaluation revealed high first-pass hepatic extraction, predicting negligible oral bioavailability. Termination at this stage saved an estimated $50 million in preclinical and clinical development costs.
Strategic Advantages
- Cost Reduction: Early identification of ADME liabilities avoids resource-intensive investments in compounds with inherent flaws, such as those requiring impractical dosing regimens or facing insurmountable formulation challenges.
- Regulatory Compliance: Regulatory bodies like the FDA and EMA mandate comprehensive ADME data for Investigational New Drug (IND) applications. Proactive profiling ensures compliance with guidelines (e.g., ICH S3A/S3B), streamlining the submission process and reducing review delays.
Future Trends and Challenges
The field of in vitro ADME studies is poised for transformative advancements:
- AI-Driven Predictive Models: Machine learning algorithms, trained on vast datasets of compound structures and ADME outcomes, are enabling in silico prediction of absorption, metabolism, and toxicity. These tools promise to revolutionize lead optimization by prioritizing compounds with "druggable" ADME properties early in the design cycle.
- Multi-omics Integration: Combining ADME data with genomic (e.g., CYP450 genetic polymorphisms) and metabolomic (e.g., endogenous metabolite profiles) information holds promise for personalized drug response prediction. This integrative approach may eventually allow tailoring therapies to individual patient populations based on their unique metabolic landscapes.
In vitro ADME studies are indispensable to modern drug discovery, serving as a bridge between molecular design and in vivo efficacy. By enabling early assessment of absorption, distribution, metabolism, and excretion properties, these studies enhance the efficiency of lead optimization, reduce development risks, and ensure regulatory readiness.
Technological innovations—from high-resolution mass spectrometry to PBPK modeling—continue to refine our ability to predict human PK behavior, while emerging trends like AI and multi-omics integration signal a future of increasingly precise and personalized ADME profiling. As the pharmaceutical industry navigates the complexity of novel therapeutic modalities (e.g., biologics, gene therapies), the foundational role of in vitro ADME studies in de-risking drug development will only grow more critical, driving forward a new era of safer and more effective medicines.
If you want to learn more about the transgenic mice, please refer to:
- In Vitro ADME Screening: Accelerating Drug Development
- In Vitro ADME Assays: Principles, Applications, and Protocols
- Differences and Connections Between ADME and DMPK in Drug Research
- In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insight
- In Vitro ADME and In Vivo PK Studies: A Holistic Framework for Drug Evaluation
- ADME in Toxicology: Ensuring Drug Safety and Efficacy
Let's Discuss Your Custom ADME Model Needs
Have a specific need for customized ADME research models for your drug development? At Creative Biolabs, our team of experienced scientists is ready to collaborate with you. We understand that every research project is unique, and we're dedicated to providing tailored solutions that precisely meet your requirements. Don't let standard models limit your scientific progress. Contact our experts today to discuss your project and discover how our custom ADME models can accelerate your research and drive meaningful results. Let us partner with you to take your scientific endeavors to the next level. We're eager to hear from you and help you achieve your research goals.
References
- Lu, Rong, et al. "Strategies and mechanism in reversing intestinal drug efflux in oral drug delivery." Pharmaceutics 14.6 (2022): 1131. https://doi.org/10.3390/pharmaceutics14061131
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
