DMPK vs. PKPD: Collaborative Mechanisms in Drug Development
Scientific division of labor from drug metabolism to effect expression
In the complex system of drug development, DMPK (pharmacokinetics) and PKPD (pharmacodynamics) are like a pair of "scientific twins" that jointly decode the dynamic process of drugs in the body. DMPK focuses on quantifying the "in vivo journey" of drugs - the entire process from oral absorption to renal excretion, using parameters such as half-life (t1/2) and bioavailability (F) to describe the trajectory of drug concentration changes over time; while PKPD focuses on the "drug efficacy code", revealing the dose-effect relationship between drug concentration and physiological effects (such as hypoglycemic and antibacterial) through indicators such as half effective concentration (EC50) and therapeutic window. The two are just like a combination of "recorder" and "decoder", and they are indispensable to form the core scientific framework of drug development.
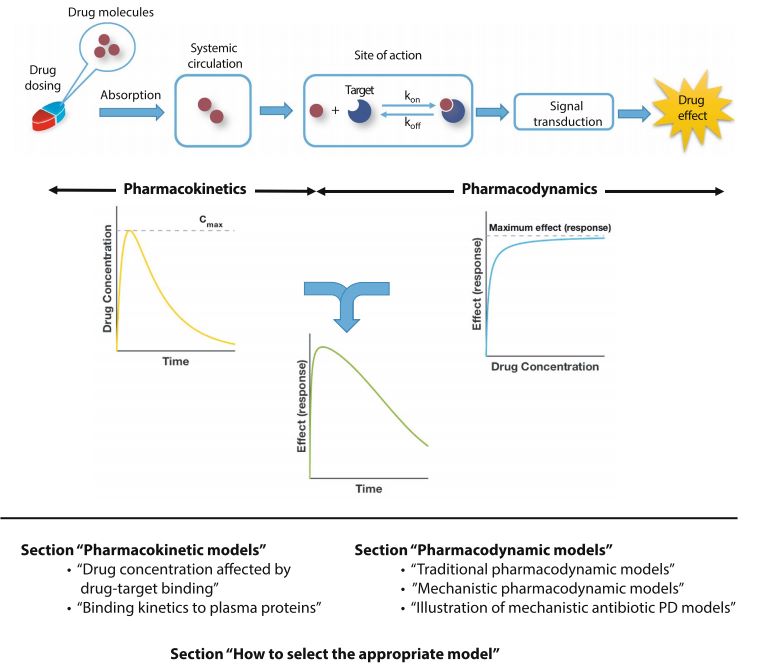 Fig. 1 Schematic overview of PK/PD modeling and review outline.1.5
Fig. 1 Schematic overview of PK/PD modeling and review outline.1.5
A dual-dimensional perspective from drug whereabouts to effect mechanism
If the drug's in vivo process is compared to "car driving", DMPK records the real-time position of the car on the road network and the fuel consumption curve, while PKPD analyzes the causal relationship between the accelerator depth and the speed and braking distance - the former describes "where" and the latter explains "how it affects".
| Dimensions | DMPK (pharmacokinetics) | PKPD (pharmacodynamics) |
|---|---|---|
| Research Core | Dynamic changes in drug concentration - time curves | Quantitative correlation of drug concentration - effect relationships |
| Key Metrics | Clearance (CL), volume of distribution (Vd), bioavailability (F) | EC50, therapeutic index (TI), maximum effect (Emax) |
| Technical means | Mass spectrometry, radiotracing, PBPK model simulation | Cell function testing, receptor binding experiments, clinical endpoint monitoring |
| Typical scenarios | Predict the proportion of a drug that enters the bloodstream after oral administration (e.g., first-pass effect assessment) | Determine the minimum effective concentration (MIC) of an antibiotic to kill bacteria |
Table 1. The difference of DMPK and PKPD
Service you may interested in
The dynamic collaboration paradigm between DMPK and PKPD
In the development process of levofloxacin, the synergistic effect of drug metabolism dynamics (DMPK) and pharmacodynamics (PKPD) constitutes a precise scientific logic closed loop. DMPK research uses advanced technologies such as high-resolution mass spectrometry and isotope labeling to deeply analyze the "journey" of the drug in the body: its half-life is about 6 hours, which means that it takes a short time for the drug to be eliminated by half in the body; the bioavailability after oral administration is as high as 99%, which can be almost completely absorbed and utilized by the human body; and 80% of the drug is excreted in the original form through the kidneys, which indicates that the kidneys are the main way for the drug to be excreted from the body. These key data provide a solid foundation for the formulation of subsequent dosing strategies, and R&D personnel can preliminarily plan the frequency and dosage of drug entry into the body.
PKPD research focuses on the mechanism of action of the drug, and further clarifies the bactericidal secrets of levofloxacin through in vitro drug sensitivity tests, animal infection models and other means. The study found that the antibacterial effect of levofloxacin is closely related to the duration of blood drug concentration above the minimum inhibitory concentration (MIC). It is necessary to ensure that the blood drug concentration exceeds the MIC for more than 60% of the time during the dosing interval in order to effectively inhibit or kill bacteria. Based on the deep combination of DMPK and PKPD, the R&D team used population pharmacokinetic models and Monte Carlo simulation methods to derive a precise dosing regimen. Due to the short half-life, in order to ensure that the drug continues to exert its antibacterial effect in the body, it needs to be administered once every 8 hours to maintain the effective concentration; at the same time, based on detailed renal clearance data, a dose-halving adjustment strategy was formulated for patients with renal insufficiency to avoid excessive accumulation of drugs in the body and cause adverse reactions.
This dynamic collaboration model has achieved remarkable results in clinical practice. Levofloxacin has shown an efficacy of 92%, which reduces the adverse reaction rate by 35% compared with the traditional empirical dosing regimen. In addition, as the study of levofloxacin continues to deepen, researchers have also found that its distribution characteristics in different infection sites are different, which further optimizes the dosing regimen for infections in special sites. This fully demonstrates the key value of the synergy between DMPK and PKPD in optimizing the therapeutic window, and also provides a classic example for the research and development of other antibiotics.
Cancer Immunotherapy: A DMPK-PKPD Breakthrough Paradigm for Biologics
The development of the PD-1 inhibitor pembrolizumab profoundly demonstrates the significant differences between biological drugs and small molecule drugs in the DMPK-PKPD model. Compared with small molecule drugs, pembrolizumab, as a biological drug, has unique DMPK characteristics. It has a long half-life (21 days), which makes the drug's action time in the body longer; low distribution volume (about 5L), reflecting the relatively limited distribution range of the drug in the body; and it is mainly metabolized by protease degradation, and the metabolic pathway is completely different from that of small molecule drugs. These characteristics directly determine the low-frequency dosing mode of pembrolizumab every 2-3 weeks, which greatly improves the patient's medication compliance.
In PKPD research, researchers have deeply explored the mechanism of action of drugs through flow cytometry, immunohistochemistry and other technologies, and found that the efficacy of this type of drug does not depend on the absolute value of blood drug concentration, but on the occupancy of PD-1 receptors. When the receptor binding rate exceeds 80%, the immunosuppressive state of T cells can be continuously released, thereby activating the body's own immune system to fight tumor cells. In order to accurately determine the effective dose and dosing regimen of the drug, the R&D team constructed a mathematical model integrating DMPK-PKPD, combined preclinical research and early clinical trial data, and conducted a large number of simulations and analyses. Finally, the minimum effective dose was accurately calculated: a dose of 10mg/kg can maintain the receptor occupancy rate above 90% for 14 days, while a higher dose (20mg/kg) not only did not significantly improve the efficacy, but also increased the incidence of immune-related pneumonia from 5% to 12%.
This major discovery directly promoted the optimization of the dosing regimen, and finally determined to conduct clinical trials with a "200mg fixed dose once every 3 weeks" regimen. In clinical trials of melanoma patients, this regimen showed amazing efficacy, extending the median survival of patients from 10.3 months to 17.9 months, significantly improving the quality of life of patients. In addition, as the research progressed, scientists also found that the efficacy of pembrolizumab in different cancer types is closely related to factors such as PD-1 receptor expression levels and tumor microenvironment. This further expanded the understanding of the dose-effect relationship of biological drugs, opened up new research directions in the field of cancer immunotherapy, and provided a unique dose-effect relationship decoding path for the development of other biological drugs.
The art of balance in clinical practice: the dual challenges of therapeutic window and individual differences
In the clinical application of warfarin, a drug with a narrow therapeutic window, the contradiction between DMPK and PKPD needs to be resolved through precise regulation. Due to the existence of CYP2C9 gene polymorphism, the metabolic rate of warfarin in different patients can differ by up to 5 times - the half-life of wild-type patients is about 40 hours, while that of patients with *3/*3 genotype can reach 90 hours. This metabolic difference directly leads to fluctuations in blood drug concentration. From the perspective of PKPD, the range between its effective concentration (1.0-2.5μg/ml) and toxic concentration is extremely narrow. When the concentration exceeds 2.5μg/ml, the risk of bleeding increases sharply, and when it is lower than 1.0μg/ml, it cannot effectively anticoagulate. To resolve this contradiction, the clinic detects the CYP2C9 and VKORC1 genotypes and adjusts the dose individually in combination with the prothrombin time (PT-INR) indicator. Studies have shown that the gene-guided dosing regimen can shorten the time to reach the target by 47%, reduce severe bleeding events by 62%, and achieve a precise balance between metabolic dynamics and effect dynamics. For the long-half-life drug aliskiren, its 48-hour half-life and 72-hour effective blood drug concentration maintenance ability, PKPD verified that its antihypertensive effect is positively correlated with the renin activity inhibition rate, which ultimately promoted the development of a once-weekly dosing regimen, increasing patient compliance from 65% of daily dosing to 89%, and there was no significant difference in efficacy, reflecting the decisive influence of DMPK on the convenience of dosing.
Future Trends
Artificial intelligence technology is fundamentally reshaping the construction paradigm of DMPK-PKPD models. The model construction that takes 8-12 weeks to complete under the traditional model can be greatly shortened after AI intervention: input compound structure information, AI algorithm can predict DMPK parameters such as clearance rate, distribution volume, etc. through machine learning. A pharmaceutical company used this technology to screen 1,000 candidate compounds, reducing the amount of DMPK experiments by 60%, and the prediction accuracy reached more than 85%. In addition, the integration of multi-omics data gives AI a more powerful effect prediction ability - in the development of an anti-inflammatory drug, AI analyzed 100,000+ gene expression data, not only identifying the main target of the drug to inhibit COX-2, but also discovered its new mechanism of activating the NRF2 antioxidant pathway, providing clues for expanding the indications of pulmonary fibrosis. A more cutting-edge application is "virtual clinical trials". The "digital patient" model built based on individual genome, intestinal flora and other data can simulate the DMPK-PKPD characteristics of different populations. A diabetes drug used this technology to identify metabolic differences in Asian populations in advance, shortening the Phase II clinical trial cycle by 12 weeks, fully demonstrating the potential for the transformation of AI-driven drug development from "trial and error" to "precise simulation."
If you want to learn more about the DMPK, please refer to:
References
- Clarelli, Fabrizio et al. "Multi-scale modeling of drug binding kinetics to predict drug efficacy." Cellular and molecular life sciences : CMLS vol. 77,3 (2020): 381-394. https://doi.org/10.1007/s00018-019-03376-y
- Igler, Claudia et al. "Multi-step vs. single-step resistance evolution under different drugs, pharmacokinetics, and treatment regimens." eLife vol. 10 e64116. 18 May. 2021, https://doi.org/10.7554/eLife.64116
- Palmer, Mary E et al. "The importance of pharmacokinetics and pharmacodynamics in antimicrobial drug development and their influence on the success of agents developed to combat resistant gram negative pathogens: A review." Frontiers in pharmacology vol. 13 888079. 25 Jul. 2022, https://doi.org/10.3389/fphar.2022.888079
- Pichardo-Almarza, C et al. "Using a Systems Pharmacology Approach to Study the Effect of Statins on the Early Stage of Atherosclerosis in Humans." CPT: pharmacometrics & systems pharmacology vol. 4,1 (2015): e00007. https://doi.org/10.1002/psp4.7
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
