Laboratory Star Mouse C57BL/6
C57BL/6 mice play a key role in many fields such as tumor research, metabolic disease research, and neuroscience research. C57BL/6 can be called a "talented player". C57BL/6 has a complete immune system, just like a fully armed "immune army"-T cells, B cells, macrophages and other "fighters" are all available, which is called "immunocompetent" in scientific research. This feature makes them a golden model for studying the interaction between immunity and disease, especially in the field of tumor research.
C57BL/6 MHC Haplotype
From a genetic perspective, C57BL/6 mice carry the H-2b haplotype MHC molecule (major histocompatibility complex), which is composed of H-2K, H-2D and other gene groups. Homozygosity makes its MHC molecule structure highly consistent, just like the "identity tag" of the immune system, which can accurately identify "foreign invaders" and "traitors in the body." Its highly homozygous genetic background makes the experimental results stable and reliable, just like a standardized "biochip", which facilitates the reproduction of data in laboratories around the world. In tumor immunity and other studies, the presentation preference of the H-2b haplotype for specific antigenic peptides helps to accurately analyze the mechanism.
C57BL/6 Mice in Tumor Research
Scientists often transplant syngeneic tumor cells (such as B16 melanoma and Lewis lung cancer cells) into them to construct an "orthotopic tumor model." At this time, the mouse's immune system will quickly initiate defense: T cells attack tumors, macrophages clean up the "battlefield," and tumor cells will also release signals to recruit "immunosuppressive cells."
C57BL/6 Macrophage Cell Line
In tumor immunity research, the C57BL/6 macrophage cell line RAW 264.7 can be regarded as a powerful "assistant". It is derived from C57BL/6 mouse monocytic leukemia, has a clear genetic background, and perfectly retains the mouse immune characteristics. This type of cell is "not picky about the environment" and can grow rapidly using the common DMEM culture medium plus 10% fetal bovine serum, with a 24-hour adhesion rate of over 90%. What's more amazing is that it can also "transform". Under different stimuli, it can flexibly switch to the pro-inflammatory M1 type or the anti-inflammatory M2 type, helping scientists to accurately simulate the immune response in the tumor microenvironment, and is an important tool for conquering tumor problems. Through the Transwell indirect co-culture or matrix mixed direct contact model, this cell line can accurately simulate the process of macrophages being "turned on their way out" in the tumor microenvironment, such as tumor-secreted CSF-1 inducing its polarization to the pro-cancer M2 type through the PI3K/Akt pathway; combined with the C57BL/6 mouse orthotopic tumor model, it can form a complete research chain of "in vitro mechanism analysis-in vivo functional verification", which is widely used in immunotherapy drug screening (such as anti-CSF-1R antibodies), nanodrug delivery and signal pathway analysis (such as NF-κB, Stat6), and is a key bridge connecting basic research and clinical transformation.
C57BL/6 Mice in Metabolic Disease Research
Feeding C57BL/6 mice a high-fat diet is like opening a "metabolic Pandora's box" for them. In just 8-12 weeks, these little guys will undergo a transformation from "healthy mice" to "metabolic abnormality models": their weight will soar by more than 40%, their blood sugar will be out of control like a roller coaster, and lipid plaques will gradually accumulate in their blood vessels-these symptoms are almost exactly the same as human obesity, diabetes, and atherosclerosis.
Why choose them for metabolic research? Because they have "fat switches" hidden in their genes. When a high-fat diet activates these switches, fat cells will grow like balloons, and the sensitivity of muscles and liver to insulin will plummet. This pathological process that is highly similar to that of humans makes C57BL/6 the "first stop" for the development of diabetes drugs. Scientists can even simulate hereditary metabolic diseases (such as leptin-deficient obesity) in them through gene editing (such as CRISPR/Cas9).
Service you may interested in
The "Gene Editing Arena" of Neuroscience
The genetic stability of C57BL/6 mice makes them the "best script actors" in neuroscience. With the help of gene editing technologies such as CRISPR/Cas9 (Figure 1), scientists can "write" human pathogenic genes into their genes, such as the APP/PS1 gene for Alzheimer's disease and the α-synuclein mutant gene for Parkinson's disease. The brains of these "customized mice" will gradually develop lesions similar to those of human patients: amyloid protein deposition, neuronal apoptosis, and decreased motor ability. Next, I will briefly introduce several transgenic mouse models.
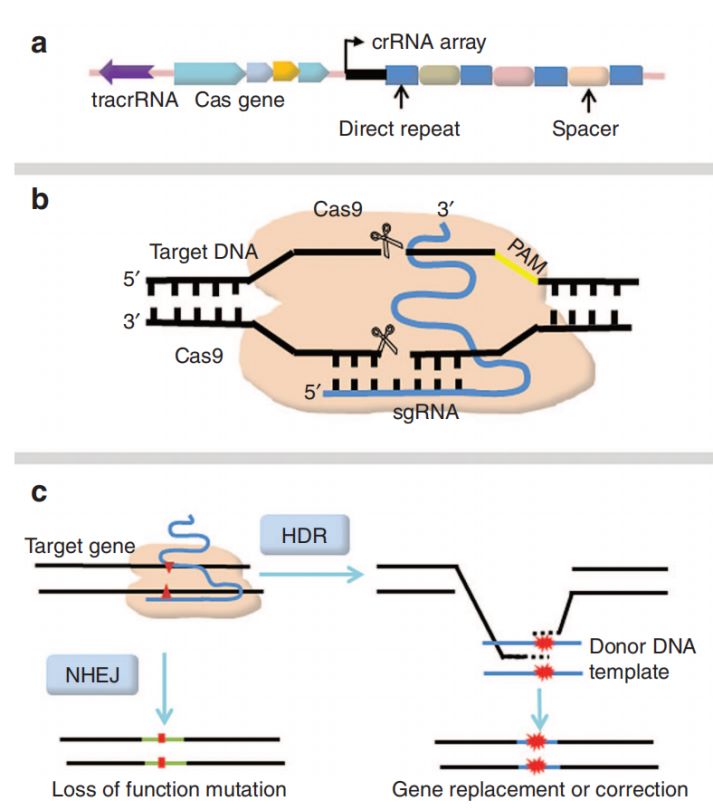 Fig. 1 Schematic representation of CRISPR-Cas9- mediated genome editing. 1,4
Fig. 1 Schematic representation of CRISPR-Cas9- mediated genome editing. 1,4
Scientific research is like a long battle to conquer major diseases, and C57BL/6 mice are a reliable "weapon" in the hands of scientists. As gene editing technologies such as CRISPR/Cas9 become more and more mature, scientists seem to have a precise "gene scalpel" that can perform precise operations on C57BL/6 mice. They have successfully cultivated various transgenic mouse models that simulate human neurodegenerative diseases by knocking out, adding, or expressing specific pathogenic genes in large quantities.
C57BL/6 Mice: The Cornerstone of Neurodegenerative Diseases
Alzheimer's disease has tortured countless patients and their families. In order to uncover its pathogenesis, scientists overexpressed pathogenic genes such as human β-amyloid precursor protein and presenilin 1 associated with Alzheimer's disease in C57BL/6 mice. Miraculously, these mice gradually developed symptoms similar to those of Alzheimer's patients, with β protein deposits in the brain, neurons slowly degenerating, and cognitive impairment.
In 2020, Sheng et al. used these transgenic mouse models of Alzheimer's disease to carefully study the relationship between β protein deposition and neuroinflammation. They found that once the β protein accumulates abnormally, it is like triggering an alarm, and the microglia in the mouse brain are immediately activated, causing neuroinflammation. Once the inflammation gets out of control, it will in turn damage neurons, causing them to die faster, and the disease will become more and more serious. Following this discovery, the researchers thought that Alzheimer's disease could be treated by controlling neuroinflammation. So they gave transgenic mice anti-inflammatory drugs, and the results were surprising-inflammation in the mouse brain was reduced, neuronal function was improved, and cognitive impairment was delayed. This research result has opened up new ideas for the treatment of Alzheimer's disease and brought hope to countless patients.
In the field of Parkinson's disease research, C57BL/6 mice also play a key role. Scientists successfully constructed a mouse model of Parkinson's disease by knocking out or changing the α-synuclein gene associated with Parkinson's disease in mice. With the help of these models, we can deeply study the causes of Parkinson's disease and explore new treatments such as stem cell therapy and gene therapy.
Interdisciplinary Contributions of C57BL/6 Mice
In fact, the "credit book" of C57BL/6 mice is far more than these. They are busy in many fields such as tumor research and metabolic disease research, driving the continuous development of life science and medicine. As scientific research technology becomes more and more advanced and research becomes more and more in-depth, I believe that C57BL/6 mice will show their talents in more fields in the future, helping us to overcome more major diseases and unlock more mysteries of life. In the starry sky of scientific research, C57BL/6 mice are like a constant North Star, silently guiding the direction of life science exploration. This small animal weighing only more than 20 grams uses the "immune code" in its genes, the "human mirror" in metabolism, and the "disease script" in nerves to build a bridge between the laboratory and the clinic. When we observe the microscopic battlefield where RAW 264.7 macrophages are "rebelled" by tumors under a microscope, or write pathogenic genes into mice on the gene editing platform, what we see is not only an experimental model, but also a microcosm of human determination and wisdom to fight against diseases.
They are "scouts" on the battlefield of tumor immunity-every game between tumor cells and immune cells provides clues for the birth of PD-1 inhibitors; they are "navigators" in the metabolic maze-every expansion of fat cells under a high-fat diet is looking for targets to reverse the fate of diabetic patients; they are "lantern bearers" in the dark night of neurodegenerative diseases-every deposition of Aβ protein in the brain is pushing the dawn of Alzheimer's disease treatment forward. These seemingly minor experiments are actually a challenge launched by humans to the essence of life, and C57BL/6 mice are the most reliable allies in this challenge.
Standing at the crossroads of technological innovation, the precise editing of CRISPR/Cas9, the three-dimensional simulation of organoid technology, and the panoramic analysis of single-cell sequencing are giving C57BL/6 mice more powerful "scientific research superpowers". In the future, they may play a more complex role in the stage of gene therapy, or reveal the code of longevity in anti-aging research, or even become a key puzzle in the frontier field of cross-species organ transplantation. But no matter how the tide of science surges, these "all-rounders" with black fur will always guard the foundation of scientific research with a stable genetic background, real physiological simulation and unlimited transformation potential.
When we close the experimental records and turn off the last laboratory light, the story of C57BL/6 mice continues to be played out in the incubator, in the gene editing instrument, and between the cells in the culture dish. They use the scale of life to measure the boundaries of human cognition; with silent dedication, they lift the weight of medical breakthroughs. On the long road of scientific research, they are not only tools, but also silent contributors, opening the door to health and hope for mankind through generations of reproduction and experiments. And this may be the warmest footnote of life science-every tiny life may become a starlight that illuminates the future of mankind.
If you want to learn more about the C57BL/6, please refer to:
References
- Dai, Wei-Jing, et al. "CRISPR-Cas9 for in vivo gene therapy: Promise and hurdles." Molecular therapy Nucleic acids 5 (2016). https://doi.org/10.1038/mtna.2016.58
- Matsuo, Naoki, et al. "Behavioral profiles of three C57BL/6 substrains." Frontiers in behavioral neuroscience 4 (2010): 1584. https://doi.org/10.3389/fnbeh.2010.00029
- Liu, Sen, et al. "Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology." Journal of Neuroinflammation 19.1 (2022): 35. https://doi.org/10.1186/s12974-022-02393-2
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
