What Are Transgenic Mice? Definition, Types & Key Concepts
Transgenic mice, engineered to carry exogenous or modified endogenous genes, have been revolutionary in biomedical research since the 1980s. Their core applications span dissecting disease mechanisms, validating treatments, and decoding gene functions. Unlike other gene-modified models, they can integrate additional genes, enabling both single-gene studies and complex multi-gene modeling. In this article, we will explore their definition, types, applications, and future directions while addressing common questions about their role in science.
What Are Transgenic Mice?
Transgenic Mice Definition
Transgenic mice are laboratory mice that carry foreign DNA (exogenous genes) stably integrated into their genome. Unlike traditional breeding methods, this DNA is introduced artificially through advanced biotechnology. The inserted gene—often from another species like humans—is designed to be inherited by offspring, enabling researchers to study its effects across generations.
The process of creating transgenic mice involves several key aspects. Scientists first carefully select the exogenous DNA they want to introduce. This DNA then randomly integrates into the mouse's genome during the early embryonic stage. Once integrated, if there is no tissue-specific promoter restricting it, the gene will be expressed in all tissues of the mouse. This unrestricted expression allows for a comprehensive study of the gene's impact on the overall physiology of the mouse. For example, some transgenic mice are engineered to overexpress cancer-causing genes, which helps in understanding the mechanisms of cancer development. Others are made to produce human proteins, providing valuable models for drug development and studying human-specific biological processes.
How Are Transgenic Mice Made? Technical Principles
The creation of transgenic mice relies on three primary methods:
- Pronuclear Microinjection: The most traditional method involves injecting linear DNA into the pronucleus of a fertilized egg. The DNA integrates randomly into the genome, with success rates typically below 10%.
- Retroviral Infection: Viral vectors (e.g., retroviruses) deliver DNA into embryonic cells, ensuring integration during cell division. However, this method is limited by the viral genome size and potential insertional mutagenesis.
- Transposon-Mediated Integration: Systems like PiggyBac use transposase enzymes to facilitate precise insertion of DNA into specific genomic sites, improving integration efficiency and reducing random mutagenesis
While transgenic mice involve random integration of exogenous DNA, knockout mice undergo targeted gene editing via homologous recombination or CRISPR/Cas9 to disrupt specific genes. Transgenic approaches are thus complementary, offering overexpression or ectopic gene expression, whereas knockout models focus on gene loss-of-function.
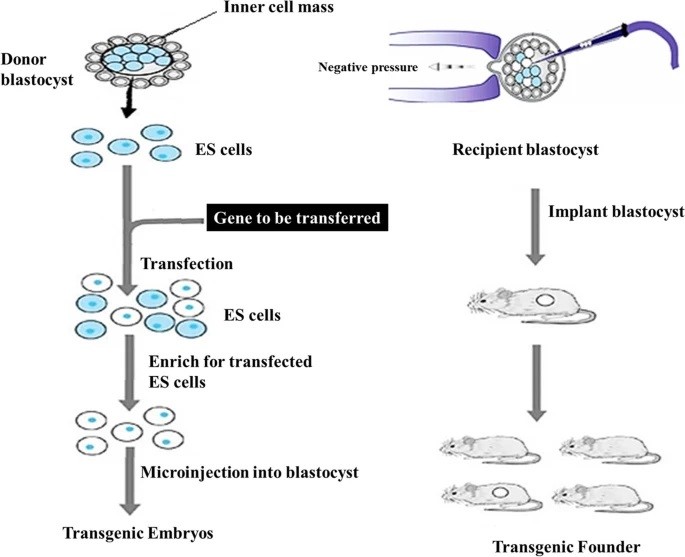 Fig. 1 The DNA microinjection technique using ES cells. 1, 3
Fig. 1 The DNA microinjection technique using ES cells. 1, 3
Core Features
- Ubiquitous Gene Expression: Transgenes are often driven by constitutive promoters (e.g., CAG promoter), leading to expression in nearly all tissues. This allows systemic analysis of gene function.
- Overexpression Phenotypes: Enhanced expression of genes like K-Ras in cancer models or APP in Alzheimer’s models recapitulates disease-related phenotypes, such as tumorigenesis or amyloid plaque formation.
Service you may interested in
Main Types of Transgenic Mice
Transgenic mice are categorized by their production methods and functional applications.
1. Classification by Technology
Random Transgenic Mice
These models rely on uncontrolled DNA insertion methods:
- Traditional Microinjection: Despite its low efficiency, this method remains widely used for generating founder mice. For example, the Tg(Tn-CAG-Luc) strain uses a transposon-mediated luciferase reporter for bioluminescent imaging.
- Retroviral Method: Efficient for delivering large DNA constructs, retroviral vectors are used in models like Tg(GFP) mice for fluorescent cell tracking.
- Transposon-Mediated: The PiggyBac system enhances integration precision, enabling single-copy insertions and reducing mosaicism.
Targeted Transgenic Mice
Advanced gene-editing tools enable precise DNA placement:
- CRISPR/Cas9: This genome-editing tool allows site-specific knock-in of transgenes (e.g., at the Rosa26 locus), overcoming the randomness of traditional methods. For instance, Tg(CRISPR-Cas9) mice enable conditional gene activation or repression.
- Embryonic Stem (ES) Cell-Based Editing: Homologous recombination in ES cells followed by blastocyst injection produces chimeric mice. This method was historically used for gene targeting before CRISPR.
2. Classification by Function and Application
Disease Models
Transgenic mice are extensively used to model a wide array of human diseases.
- Cancer Models: K-Ras transgenic mice develop lung adenocarcinomas, mimicking human oncogenic pathways.
- Neurodegenerative Disease Models: APP transgenic mice overexpress mutant amyloid precursor protein, recapitulating amyloid plaques seen in Alzheimer’s disease.
- Metabolic Disorder Models: Lepob/ob mice, deficient in leptin, exhibit obesity and insulin resistance, modeling type 2 diabetes.
Humanized Mice
These mice express human genes or tissues, enabling studies of human-specific biology. For example:
- HLA Transgenic Mice: Express human leukocyte antigens (HLAs) for immunological research.
- Hu-NSG Mice: Engrafted with human hematopoietic stem cells, they model human immune responses.
Reporter Gene Mice
These mice express genes that encode easily detectable proteins, such as fluorescent proteins or luciferase.
- Fluorescent Markers: Villin-EGFP mice express green fluorescent protein (GFP) in intestinal epithelial cells, aiding lineage tracing.
- Luciferase Mice: Tg(Tn-CAG-Luc) mice use luciferase for non-invasive imaging of gene expression or tumor growth.
These categories often overlap – for instance, a disease model might include a reporter gene to track tumor cells, or a humanized mouse might be used as a disease model. Collectively, transgenic mice whether designed for disease, humanization, or reporting, have become a foundational toolset in biomedical research.
Key Concepts in Transgenic Mouse Research
This section covers some fundamental concepts to understand how transgenic mice are made, named, and used, as well as their advantages and limitations in research.
Production of Transgenic Mice: Process and New Techniques
Creating transgenic mice involves five key steps:
- Gene Construct Design: Designing DNA vectors with promoters, transgenes, and selection markers (e.g., antibiotic resistance).
- Embryo Manipulation: Microinjecting DNA into fertilized eggs or infecting embryos with viral vectors.
- Embryo Transfer: Implanting manipulated embryos into pseudopregnant foster mothers.
- Genotype Identification: Screening pups for transgene integration via PCR or Southern blotting.
- Line Establishment: Breeding founder mice to establish stable transgenic lines.
Transgenic mouse technology has advanced significantly with the optimization of the PiggyBac transposon system and the emergence of CRISPR/Cas9. PiggyBac offers efficient, single-copy gene integration, ensuring stable expression. CRISPR/Cas9 enables precise gene editing, shortens development time from months to weeks, and allows multi-gene manipulation for complex disease modeling. These breakthroughs enhance experimental accuracy, expand research scope from single-gene to complex disease studies, and accelerate translational medicine by creating more human-relevant models, as seen in luciferase-expressing PiggyBac mice and CRISPR-engineered Alzheimer's models.
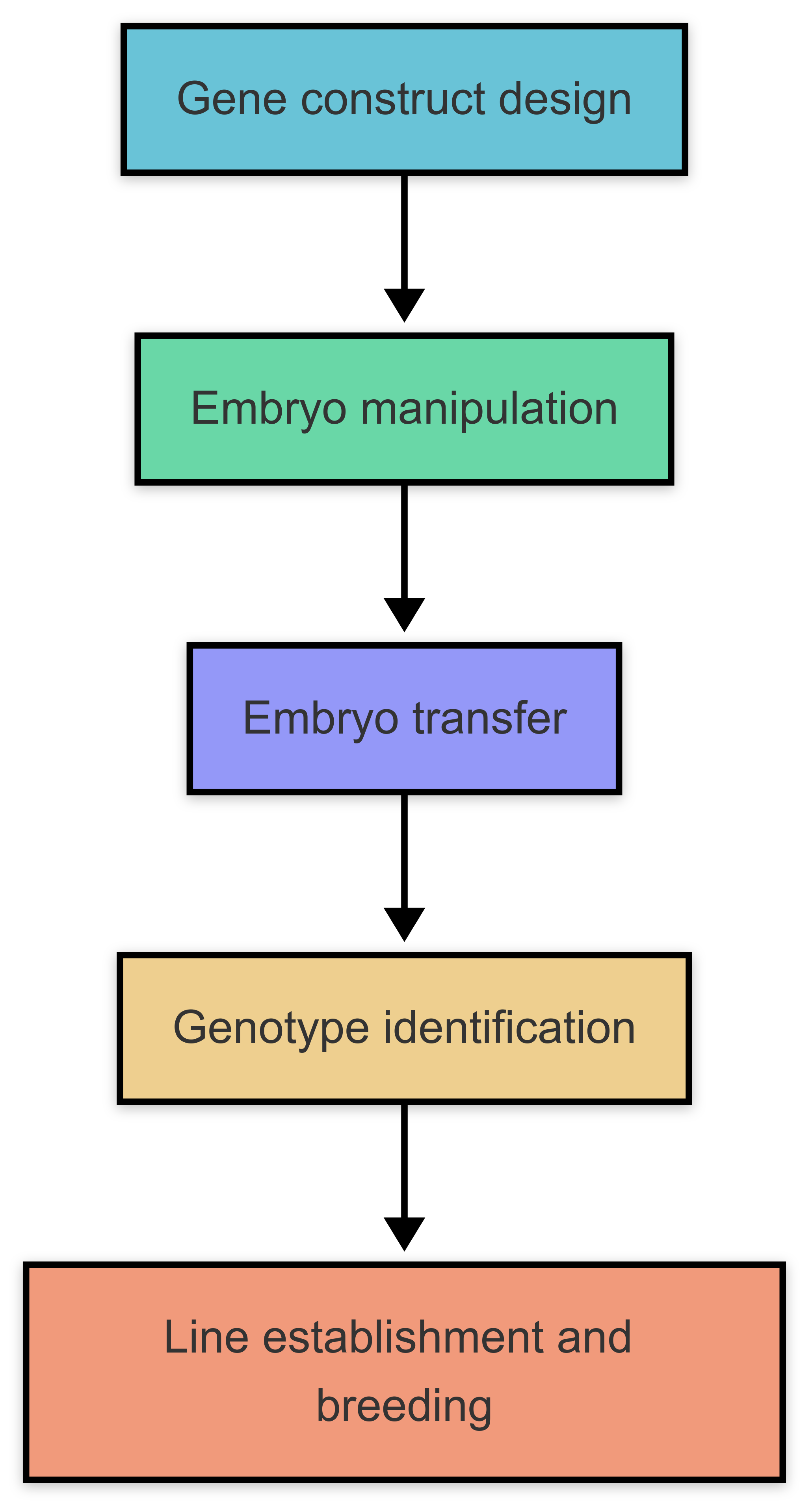 Fig. 2 Flow chart for preparation of transgenic mice.
Fig. 2 Flow chart for preparation of transgenic mice.
Nomenclature of Transgenic Mouse Lines
Transgenic mice naming follows a systematic set of rules. The name always begins with "Tg" to signify a transgenic animal. Inside the name, the gene construct is described, listing the promoter first, followed by the transgene symbol. For example, "Tg(CAG-GFP)" means the CAG promoter controls the expression of green fluorescent protein.
When specific insertion techniques are used, such as transposons or CRISPR, their abbreviations are added before the promoter. To differentiate between strains, unique numbers are assigned to different integration events of the same construct. For genes from other species, a three-letter code indicating the species is added before the gene symbol.
Tissue-specific expression is shown by directly including the relevant promoter in the name. This naming system organizes information about the technique, promoter, gene, species, and strain. It ensures accurate identification of transgenic models in research, avoids misunderstandings, and helps with repeating experiments.
Applications
Transgenic mice are highly valuable in scientific research, with applications in three main areas.
- Basic Research: In gene function study, they verify gene functions by knocking in or over-expressing genes and observe phenotype changes. Reporter genes help track gene expression patterns.
- Disease Studies: For human diseases, transgenic mice model various conditions. They express oncogenes for cancer research, replicate amyloid plaque deposition in Alzheimer's, induce metabolic-pathway defects for diabetes study, and use human-like immune components for immunological research.
- Drug Development: In drug development, they validate drug targets, assess drug efficacy in disease models, and predict drug toxicity using humanized models. Acting as "living laboratories", transgenic mice bridge basic and clinical research. However, combining with techniques like organoids can offset species-difference drawbacks.
Advantages and Limitations of Using Transgenic Mice
The use of transgenic mice in research offers several significant advantages but also comes with certain limitations that researchers must consider.
- Advantages: Transgenic mice offer significant advantages in life science research. They can precisely mimic human diseases. For example, Alzheimer's transgenic mice with mutant human genes replicate the disease's key symptoms, helping study pathogenesis and develop treatments. In gene function research, overexpression or knockout transgenic mice clarify gene roles, like PTEN knockout mice revealing its tumor-suppressing function. They also speed up drug R&D by simulating human drug responses, as seen in cardiovascular drug tests using hypertensive transgenic mice.
- Limitations: Transgenic mice, crucial in scientific research, come with notable limitations. Technically, traditional methods relying on random gene integration face issues. The position effect, due to the influence of the insertion site's genomic environment, causes unstable expression of exogenous genes, and epigenetic silencing may gradually suppress transgene expression over time. Moreover, random insertions risk disrupting endogenous gene functions, generating unexpected phenotypes that cloud experimental results. Regarding disease models, vast cross-species physiological disparities exist. Mice and humans differ in organ structure, metabolism, immune systems, and lifespan, with gene regulatory networks also being species-specific. For instance, Alzheimer's disease mouse models lack key features like neurofibrillary tangles despite forming amyloid plaques, and complex multi-gene diseases are hard to fully mimic. These limitations drive the progress of gene-editing technologies like CRISPR and multi-species research systems to improve disease model accuracy and translational value.
Future Directions
The field of transgenic mouse research is continuously evolving, with several exciting future directions aimed at enhancing the precision and relevance of these powerful models.
- Technological Innovations: The combination of CRISPR/Cas9 technology with single-cell sequencing represents a promising path for future growth. Researchers can execute precise genome alterations at designated loci through this combination while simultaneously evaluating these changes at the single-cell level. The approach delivers unparalleled understanding of gene functions and regulatory systems alongside diverse cellular reactions to genetic alterations. In vivo tracking of edited cells alongside integration of single-cell omics technologies will likely become future applications for enhanced understanding of cellular processes.
- Model Optimization: Another really important thing we're focusing on is making existing models better, especially when it comes to developing fancier humanized mice. What this means is creating mice that have a more complete and working human immune system. That includes getting human hematopoietic stem cells and lymphoid tissues to graft better, and also making sure they express human-specific cytokines and immune factors. These new-fangled models are going to be super useful. We can use them to study infectious diseases that are unique to humans, autoimmune problems, and also for doing pre-clinical tests on immunotherapies and vaccines in a way that's more like what happens in the human body.
If you want to learn more about the transgenic mice, please refer to:
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Conclusion
Transgenic mice are now a really essential tool in biomedical research. They offer a super flexible platform for studying how genes work, creating models of human diseases, and coming up with new treatments. Before, traditional methods just randomly stuck transgenes in. But now, with cool things like using CRISPR/Cas9 for targeted gene editing and transposon systems, we can make these mouse models way more precisely and efficiently. Also, being able to create humanized mice and reporter gene mice has made them even more useful. Sure, there are some drawbacks. For example, the expression might not be stable, and they might not fully copy human diseases. But with new tech constantly being developed and people really paying attention to ethical issues, transgenic mice will probably become even more valuable. They'll help us answer important biological questions and deal with the biggest problems in human health.
Act Now to Transform Your Ideas into Reality
If you have unique insights into transgenic model development, today. Let us handle the technical complexities while you focus on discovery. Together, we can push the boundaries of biomedical research.
References
- Shakweer, W. M. E., et al. "A review of transgenic animal techniques and their applications." Journal of Genetic Engineering and Biotechnology 21.1 (2023): 55. CCBY4.0 https://doi.org/10.1186/s43141-023-00502-z
- Li, Limei, et al. "PiggyBac transposon-based polyadenylation-signal trap for genome-wide mutagenesis in mice." Scientific Reports 6.1 (2016): 27788. https://doi.org/10.1038/srep27788
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
