Humanized Transgenic Mice: Bridging Animal Models & Human Disease Studies
Traditional animal models (such as ordinary mice) have significant genetic, physiological and immune differences from humans, making it difficult to accurately simulate human diseases and predict drug responses. For example, nearly 90% of drugs that are effective in mouse experiments have failed clinical trials due to differences in cross-species responses, which greatly wastes research and development resources and delays treatment progress. In order to break through this bottleneck, humanized transgenic mice are implanted into human genes, cells or tissues through gene editing technology to build animal models that can simulate human biological characteristics. Its core advantage lies in narrowing the species divide-it can not only accurately reproduce the pathological mechanism of specific diseases, but also simulate the human drug metabolism process, thus becoming a key bridge for the transformation of basic research into clinical application.
Humanized transgenic mice have significantly optimized pre-clinical evaluation of drug efficacy and safety by improving the match between disease models and real humans, for example, demonstrating irreplaceable roles in areas such as targeted cancer therapy and immune disease mechanism analysis. At the same time, as a core tool in translational medicine, it significantly shortens the new drug research and development cycle, reduces the risk of failure due to species differences, and provides a pre-verification platform for personalized treatment plans. Future research needs to further refine the classification of models (such as humanization of the immune system, reconstruction of tumor microenvironment, etc.), explore its application potential in complex diseases such as neurodegenerative diseases and infectious immunity, and ultimately promote the transformation of laboratory results into clinical treatment. Substantive leap forward.
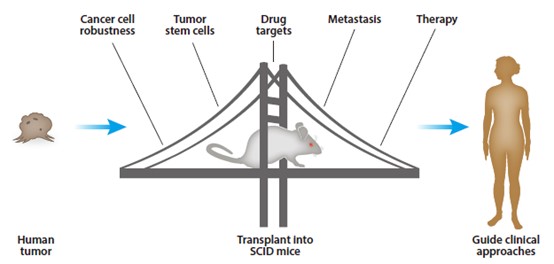 Fig. 1 Humanized mice in cancer biology and therapy. 1,2
Fig. 1 Humanized mice in cancer biology and therapy. 1,2
Main types of humanized transgenic mice
Human ACE2 transgenic mice
hACE2 is a key receptor for the new coronavirus (SARS-CoV-2) to invade host cells. By building K18-hACE2 transgenic mice, scientists successfully simulated the infection process of the virus in the lungs and central nervous system, revealing the potential mechanism of neural invasion. However, overexpressing hACE2 may lead to non-physiological severe diseases, so a new generation of low-copy hACE2 mice (1-hACE2-Tg) and genome-based AACE2-GR mice are closer to human mild infections and have become the preferred model for vaccine and antiviral drug evaluation.
Fully Human Antibody Transgenic Mice
The immunoglobulin (Ig) gene locus of these mice has been completely replaced with a human version, producing diverse and high-affinity fully human antibodies. For example:
The anti-EGFR monoclonal antibody Panitumumab and the anti-RANKL monoclonal antibody denosumab were both developed through such models and successfully marketed.
In the COVID-19 pandemic, using Trianni mouse mRNA immunization technology, neutralizing antibodies against Omicron were screened in just 7 days.
Advantages: Avoid the immunogenicity risks of traditional humanized antibodies and accelerate the development of antibody drugs.
HLA Transgenic Mice
Human leukocyte antigen (HLA) is a key molecule for T cells to recognize antigens. HLA-A2 and HLA-DR4 transgenic mice are prominent in vaccine development and autoimmune disease research:
- HLA A2 transgenic mice: As a key subtype of human major histocompatibility complex (MHC) class I molecules, HLA A2 has shown important value in the research fields of immune oncology, transplant immunity, and autoimmune diseases. In the field of immuno-oncology, this mouse can simulate the expression pattern of HLAA2 molecules on the surface of human tumor cells, building an ideal model for in-depth exploration of tumor antigen presentation and the recognition and killing mechanisms of T cells on tumor cells, and further for research and development. Effective tumor immunotherapy strategies provide support. In transplant immunity research, it can be used to analyze the mechanism of transplant rejection and explore corresponding preventive measures.
- HLA DR4 transgenic mice: HLA DR4 belongs to MHC class II molecules and is closely related to many autoimmune diseases such as rheumatoid arthritis. This mouse can simulate the expression of human HLA-DR4 molecules and is of great value in the study of HLA-DR4-related autoimmune diseases such as rheumatoid arthritis. Through this model, researchers can conduct in-depth research on the pathogenesis of diseases and screen therapeutic drugs for specific pathogenic links.
Human FcRn Transgenic Mice
Neonatal Fc receptors (FcRn) significantly affect the half-life of antibody drugs by regulating the recycling mechanism of IgG antibodies. Human-derived FcRn transgenic mice (such as the Tg32 strain) have become a key tool in the development of antibody drugs because they accurately simulate the biological functions of human FcRn. In terms of optimizing antibody efficacy, researchers modified the Fc segment structure of Herceptin to enhance its binding ability to FcRn, and successfully extended its half-life to 3 times in a mouse model, providing a basis for reducing clinical medication frequency. For the treatment of autoimmune diseases, this model has verified the effectiveness of anti-FcRn therapy in rheumatoid arthritis studies-significantly accelerating the clearance of pathogenic autoantibodies by blocking the FcRn-mediated antibody recovery pathway. In addition, based on quantitative tissue FcRn expression data from Tg32 mice, the researchers constructed a physiological pharmacokinetic (PBPK) model that accurately simulated the distribution and metabolism of antibodies in humans, greatly improving the prediction reliability of drug dose and efficacy in preclinical studies.
Other Human Transgenic Mice
In addition to the above types, there are many other types of humanized mouse models, such as mice carrying human cytokines, chemokines, or disease-related genes. For example, mice carrying the human interleukin- 2 (IL-2) gene can be used to study IL-2-related immune regulatory mechanisms and diseases; mice carrying Alzheimer's disease-related genes can be used to simulate Alzheimer's disease. Pathological process. These models have unique value in specific disease research and provide diverse tools for in-depth discussion of the mechanism of disease occurrence and development.
Application of humanized transgenic mice in human disease research
Infectious Disease Research
During the COVID-19 epidemic, human ACE2 transgenic mice assisted SARS-CoV-2 research and provided critical data for vaccine and drug development. Not only that, other types of humanized mice have also performed well in the research of infectious diseases such as SARS, influenza, and AIDS. For example, mice carrying human HIV receptors can be used to study the HIV infection process and the development of anti-AIDS drugs.
Tumor Immunology Research
HLA transgenic mice can simulate the expression of human MHC molecules. In the research on tumor immunotherapy mechanisms and the development of new therapies, they can accurately evaluate the efficacy and mechanisms of tumor vaccines, immune checkpoint inhibitors, etc. Humanized immune system mice can rebuild the human immune system, provide a realistic model for evaluating human responses to immune checkpoint inhibitors, and help develop efficient tumor immunotherapy strategies.
Research on autoimmune diseases
HLADR4 transgenic mice are of great significance for the study of autoimmune diseases such as rheumatoid arthritis and multiple sclerosis. Researchers have used this to identify key pathogenic factors and signal pathways and develop targeted therapeutic drugs. In addition, humanized mouse models carrying human self-antigen genes also help explain disease pathogenesis.
Neurodegenerative diseases
In the research of neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease, humanized mice have broad prospects. After introducing human disease-related genes, mice can simulate human-specific pathological characteristics, such as amyloid deposition, neuron apoptosis, etc., providing an ideal model for studying disease mechanisms and developing therapeutic drugs.
Other disease areas
Humanized transgenic mice have many applications in cardiovascular and metabolic disease research. For example, mice carrying human hyperlipidemia genes can help research on the pathogenesis of cardiovascular diseases and the development of lipid-lowering drugs; mice carrying insulin resistance genes can help explore the mechanism of metabolic diseases such as diabetes and develop therapies.
The value of humanized transgenic mice in translational medicine
The physiological and pathological characteristics of humanized transgenic mice are closer to those of humans and can accurately simulate the human body's response to drugs. During drug development, pre-clinical research using this model can improve the accuracy of drug safety assessment and pharmacodynamic evaluation, reduce the failure rate of research and development caused by differences between animals and humans, improve the success rate of research and development, and reduce costs.
Humanized mice play a key role in the development of new therapies such as antibodies, cells, and genes. For example, fully human antibody transgenic mice accelerate the development of fully human antibody drugs; humanized immune system mice provide realistic models for cell therapy evaluation; mice carrying human disease genes become ideal carriers for gene therapy. In addition, it has great potential in the development of personalized medical strategies, building models based on patients 'genetic characteristics and helping formulate personalized treatment plans.
Compared with traditional animal models, humanized transgenic mice have significant advantages in disease simulation and drug response prediction. Traditional models are difficult to accurately simulate human disease processes and drug responses due to genetic and physiological differences. Humanized models can accurately reflect human biological characteristics and provide unique assistance for the study of human-specific disease mechanisms.
Conclusion and prospect
Humanized transgenic mice build a bridge between animal models and human disease research, provide assistance for research in multiple fields such as infectious diseases and tumors, and promote the exploration of new drugs and therapies.
But this technology still faces challenges. Technically, it is difficult and costly to build complex humanized models, and the degree of humanization needs to be improved; ethically, the application of gene editing technology needs to be strictly regulated and supervised. With the development of gene editing technology, humanized transgenic mouse technology will move towards deep humanization and integration with multiple organ chips, bringing new breakthroughs to disease research.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Humanized Mice: Advancing Your Research
Looking to elevate your preclinical research with the power of humanized mouse models? At Creative Biolabs, our dedicated experts are ready to guide you through the possibilities. Unlock deeper insights into human biology and disease by leveraging our customized humanized mice. Contact us today to explore how our tailored solutions can significantly advance your experimental outcomes.
References
- Walsh, Nicole C., et al. "Humanized mouse models of clinical disease." Annual Review of Pathology: Mechanisms of Disease 12.1 (2017): 187-215. https://doi.org/10.1146/annurev-pathol-052016-100332
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
