Transgenic Mice vs. Knockout Mice: Understanding the Differences & Research Benefits
In the scope of contemporary life science exploration, transgenic mice and knockout mice, as two widely used model organisms, play a key role in many fields such as gene function analysis, disease model construction, and drug research and development. Although both are the results of gene editing technology, there are obvious differences in core concepts, technical routes, and application scenarios. This paper intends to comprehensively analyze and compare these two models from multiple dimensions, aiming to help researchers understand more deeply and accurately select suitable models.
Comparison of core concepts
Definition and technical principles of transgenic mice
Transgenic mice are a type of model organism that randomly inserts foreign genes (such as human genes) into the mouse genome through techniques such as microinjection or virus vector. Its technological evolution has gone through three important stages:
1.0 Times: Pronucleus microinjection This is the earliest developed transgenic technology. It achieves random integration of genes by injecting foreign DNA directly into the pronucleus of a mouse fertilized egg. The success rate of this method is about 30-40%. Although the operation is relatively simple, due to random integration, it may lead to uncertainty in the position of foreign genes in the genome, thus affecting the expression effect.
2.0 Times: The transposon system is represented by the PiggyBac transposon system. This technology has significantly improved the efficiency of transgene and increased the efficiency of BAC to 70%. Transposons can carry foreign genes to leapfrog integrate in the genome. Compared with pronuclear microinjection, their integration efficiency and stability are greatly improved.
3.0 Times: Targeted integration with the development of gene editing technology, the targeted integration of foreign genes in the mouse genome has been achieved, such as the Rosa26 locus. This technology can accurately control the insertion position of foreign genes, achieve controlled expression, avoid the position effect caused by random integration, and provide strong support for building a more stable transgenic model.
Definition and technical principles of knockout mice
Knockout mice are model organisms that inactivate specific genes through homologous recombination or CRISPR technology. Its key technological breakthroughs mainly include the following aspects:
Embryonic stem cell (ES cell) targeting This is the traditional gold standard method for gene knockout. By carrying out homologous recombination in embryonic stem cells, the target gene is knocked out, and then the modified embryonic stem cells are injected into mouse blastocysts. Finally, gene knockout mice are obtained. This method has a long cycle, usually taking 6-12 months, but has high accuracy and stability.
The emergence of CRISPR/Cas9 directly fertilized egg editing CRISPR/Cas9 technology has greatly shortened the gene knockout cycle, shortening it to 3 months, with an efficiency of more than 90%. This technology achieves gene knockout or modification by designing specific sgRNAs to guide Cas9 nuclease to target gene loci. It is simple to operate and efficient, making it the current mainstream technology for gene knockout.
Conditional knockout technology uses the Cre-loxP system to achieve tissue-specific gene silencing. This technology can knock out target genes in specific tissues or cell types and at specific time points, avoiding the possibility of systemic knockout. Fetal deaths or other side effects provide a powerful tool for studying the tissue-specific functions of genes.
Table 1 Comparison of core differences
| Transgenic Mice | Knockout Mice | |
|---|---|---|
| Gene Manipulation Direction | Foreign gene addition (Gain-of-function) | Endogenous gene deletion (Loss-of-function) |
| Integration Site | Random (approximately 70% non-coding regions) | Targeted to a specific gene locus |
| Main Techniques | Microinjection, Transposon, Site-specific Integration | Homologous Recombination, CRISPR |
| Typical Applications | Overexpression disease models, Drug target validation | Gene function research, Essential gene analysis |
Service you may interested in
In-depth analysis of technical path and efficiency
Comparison of gene editing efficiency
- Transgenic success rate: The Founder's positive rate of traditional microinjection is 30-40%; the PiggyBac transposon system increases the BAC transgene efficiency to 70%; and the Rosa26 site-specific integration technology has extremely high expression stability, with an efficiency exceeding 95%. It can be seen that with the continuous advancement of technology, the construction efficiency and stability of transgenic mice have been significantly improved.
- Knockout efficiency: The efficiency of CRISPR/Cas9 directly fertilized egg editing technology exceeds 90%. Compared with traditional embryonic stem cell targeting methods, it has obvious efficiency advantages and greatly accelerates the construction of gene knockout mice.
Technical limitations
- Transgenic mice
Position effects lead to abnormal expression: Since foreign genes are randomly integrated into the mouse genome, about 30% of the models require multi-strain validation to rule out the effect of position effects on gene expression.
Inability to accurately control copy number: The inherent defects of microinjection make it difficult to accurately control the copy number of foreign genes, which may affect the expression level and functional research of genes.
15% gene knockout causes embryo death: Systemic knockout of certain genes can cause embryo death during development, which limits the functional research of these genes and needs to be combined with conditional knockout technology.
Systemic knockout may mask tissue-specific functions: Systemic knockout affects target genes in all tissues and cells and may mask specific functions of genes in specific tissues, so conditional knockout techniques need to be combined to achieve tissue-specific gene knockout.
Analysis of application scenarios in disease research
Typical applications of transgenic mice
Overexpression disease models build pathological models by artificially regulating the expression levels of specific genes, providing key experimental tools for disease mechanism research and drug development. For example, in Alzheimer's disease research, transgenic mice that overexpress human APP and PS1 genes can simulate core pathological characteristics such as amyloid plaque deposition and neuron death, becoming a basic model for analyzing pathogenesis and testing drugs; In the field of cancer, breast cancer models that overexpress the HER2 gene reveal the role of this receptor in tumorigenesis and promote the development of precise drugs targeting HER2. By accurately reproducing the hallmark phenotype of the disease, such models build a bridge from the exploration of molecular mechanisms to the verification of treatment strategies.
Drug testing platform: Humanized CYP450 models are used for metabolic research. CYP450 enzyme is a key enzyme in drug metabolism. By transferring the human CYP450 gene into mice and building a humanized model, drugs in humans can be more accurately evaluated. Metabolism process and safety.
Core values of knockout mice
Decoding gene function:The knockout mouse model is an important tool for analyzing gene functions and disease mechanisms. Its core value is reflected in two aspects: at the basic research level, the biological role of genes can be accurately revealed by targeting specific genes, such as the L1CAM gene knockout research clarified the key functions of this adhesion molecule in nerve synapse formation and neuron migration, and deepened the understanding of nervous system development; At the level of translational medicine, knockout models can directly simulate disease phenotypes. For example, ApoE knockout mice spontaneously form atherosclerotic plaques due to lipid metabolism disorders, becoming a gold model for studying pathological mechanisms and drug screening. These models provide an irreplaceable research platform for deciphering gene functions and exploring disease targets.
Drug target verification: ANGPLT6 knockout and overexpression jointly verify anti-obesity targets. By knocking out and overexpressing the ANGPLT6 gene, its role in fat metabolism and obesity is studied, providing potential targets for the development of anti-obesity drugs.
Joint application cases
Study on the Mechanism of Diabetes
Liver specific overexpression of IKKβ induces insulin resistance: Specific overexpression of the IKKβ gene in the liver can induce insulin resistance and mimic liver lesions in type 2 diabetes.
Combined with Slc2a4 knockout to verify the glucose transport mechanism: Slc2a4 is a glucose transporter 4. Knockout of this gene can further verify the role of glucose transport in insulin resistance. By jointly applying these two models, we can deeply study the pathogenesis of diabetes.
Neurological disease research
NCX1 overexpression/knockout bidirectional regulates stroke susceptibility: NCX1 is a sodium-calcium exchanger. Overexpression and knockout of NCX1 gene can increase and decrease stroke susceptibility respectively. Through bidirectional regulation research, reveal the mechanism of action of NCX1 in stroke occurrence.
Model selection decision system
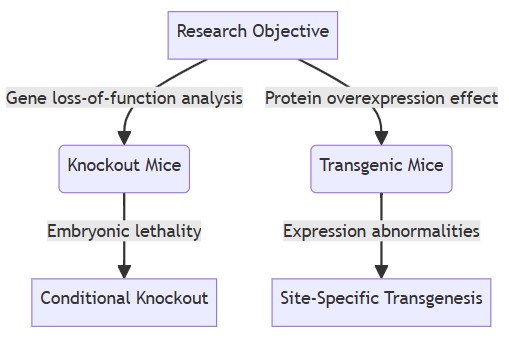 Fig. 1 Decision tree for selecting appropriate mouse models.
Fig. 1 Decision tree for selecting appropriate mouse models.
When selecting transgenic mice or knockout mice, the decision needs to be based on specific research goals:
- Gene loss of function analysis: If the goal of the study is to explore the functional changes of a gene after deletion, knockout mice are a better choice. Especially when it is necessary to study the tissue-specific functions of genes, conditional knockout techniques can accurately achieve this goal.
- Protein overexpression effect: If research needs to observe the impact of overexpression of foreign genes or own genes, such as studying the relationship between overexpression of a certain protein and disease occurrence, transgenic mice are more appropriate. Site-specific transgenic technology can achieve controlled expression of foreign genes and improve the stability of models.
- Embryonic Lethal: When systemic knock-out of a target gene leads to the death of the embryo, conditional knock-out technology must be used to knock out the gene in a specific tissue or development stage to avoid embryo death, thereby studying the function of the gene in adults.
- Abnormal expression: Transgenic mice may have abnormal expression due to random integration of foreign genes, which requires multi-strain verification; while knockout mice need to pay attention to avoiding embryonic mortality and masking of tissue-specific functions during the construction process.
To sum up, transgenic mice and knockout mice each have their own unique advantages and application scenarios. Researchers should select the appropriate model or jointly apply the two models based on specific research goals, genetic characteristics and experimental needs to obtain more accurate and reliable research results. With the continuous development of gene editing technology, these two models will play a more important role in life science research, providing more ideas and methods for the diagnosis, treatment and prevention of diseases.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Find your ideal model
If you are confused about the choice of experimental models and don't know which model best suits your research needs, please contact Creative Biolabs today! Our experienced team of experts will have an in-depth understanding of your experimental goals, tailor the most suitable model solution for you, and help you easily start your research path.
Reference
- Miller, R. Lance. "Transgenic mice: beyond the knockout." American Journal of Physiology-Renal Physiology 300.2 (2011): F291-F300. https://doi.org/10.1152/ajprenal.00082.2010
For Research Use Only.
