Differences & Connections Between ADME & DMPK in Drug Research
Introduction: Setting the Stage for Drug Development
The Crucial Role of Pharmacokinetics in Drug Discovery
Understanding how a drug interacts with the human body—its absorption, distribution, metabolism, and excretion (ADME)—lies at the core of modern drug discovery. The journey from a novel chemical entity to an approved therapeutic is fraught with complexity, with over 90% of candidates failing in clinical trials due to issues like poor efficacy or safety. Pharmacokinetics, the study of how the body processes drugs, serves as a critical lens through which researchers evaluate a compound's potential.
Defining ADME and DMPK: Initial Overview
ADME refers to the fundamental processes governing a drug's fate:
- Absorption: How a drug enters the bloodstream.
- Distribution: How it spreads to tissues and organs.
- Metabolism: How it is chemically modified.
- Excretion: How it is eliminated from the body.
DMPK (Drug Metabolism and Pharmacokinetics), conversely, is an integrated discipline that merges ADME insights with quantitative modeling to predict and optimize drug behavior. While ADME focuses on individual processes, DMPK applies this knowledge strategically to advance drug candidates through development.
Why Understanding ADME and DMPK Matters
Suboptimal ADME/DMPK properties are a primary cause of late-stage clinical failures, costing billions of dollars. By characterizing these properties early, researchers can triage candidates, minimize risks, and enhance the likelihood of developing safe, effective therapies.
Target Audience and Article Goal
This article aims to clarify the nuances between ADME and DMPK for researchers, students, and industry professionals, offering the foundation to apply these concepts in drug discovery.
Deep Dive into ADME Properties: The Fundamentals of Drug Fate
Absorption: Getting into the System
Absorption determines a drug's bioavailability—the fraction reaching the systemic circulation. Key mechanisms include passive diffusion (e.g., through intestinal epithelium) and active transport (mediated by transporters like P-glycoprotein). Factors such as solubility, permeability, and first-pass metabolism in the liver profoundly impact oral absorption. For instance, low solubility often correlates with poor oral bioavailability, driving the need for formulation optimization.
Distribution: Where Does the Drug Go?
Distribution reflects how a drug spreads from blood to tissues, influenced by plasma protein binding (e.g., to albumin), tissue affinity, and barriers like the blood-brain barrier. High plasma protein binding can reduce a drug's active concentration, while CNS-penetrant drugs must overcome lipid membranes to reach the brain.
Metabolism: Transformation within the Body
The liver and intestines are primary sites of metabolism. Phase I reactions (e.g., oxidation by CYP enzymes) introduce functional groups, while Phase II reactions (e.g., conjugation by UGTs) increase water solubility for excretion. Genetic variations in enzymes like CYP2D6 can lead to interindividual differences in metabolism, affecting efficacy or toxicity. Metabolites may be active (e.g., contributing to therapeutic effects) or toxic (e.g., causing adverse reactions).
Excretion: Eliminating the Drug
Renal excretion (via glomerular filtration and tubular secretion) and biliary excretion (via the liver to intestine) are major routes. Transporters like P-glycoprotein influence renal clearance, while impaired renal function in patients can prolong drug half-life, necessitating dose adjustment.
Case Studies in ADME Action
Consider a hypothetical compound with poor intestinal permeability: Early ADME studies identifying this issue could prompt structural modifications to enhance solubility, thereby improving oral bioavailability before advancing to preclinical trials.
Exploring DMPK Properties: A Broader Perspective
Defining DMPK: Beyond Individual Parameters
DMPK is not merely a sum of ADME processes but a quantitative framework for predicting drug behavior. It emphasizes modeling to translate preclinical data to human pharmacokinetics (PK), guiding dose selection and clinical trial design.
Pharmacokinetics: The Quantitative Backbone
PK describes how drug concentrations change over time, defined by parameters such as:
- AUC (Area Under the Curve): Total exposure.
- Cmax: Peak concentration.
- Half-life: Time to reduce concentration by 50%.
- Clearance: Rate of drug elimination.
PK modeling (e.g., compartmental models) helps predict how dose adjustments affect exposure, crucial for optimizing therapeutic windows.
Drug Metabolism: The Core of DMPK
Drug metabolism studies identify metabolic pathways (e.g., which CYP enzymes are involved) and potential drug-drug interactions (DDIs). For example, if a drug inhibits CYP3A4, it may increase the exposure of co-administered drugs metabolized by the same enzyme, raising toxicity risks. In vitro assays (e.g., microsomal stability tests) and in vivo studies in animals characterize metabolic profiles.
Integrating DM and PK
Metabolism directly influences PK: Fast metabolism may shorten half-life, while active metabolites can extend efficacy. Physiologically Based Pharmacokinetic (PBPK) modeling integrates organ-specific ADME data (e.g., liver blood flow, enzyme expression) to predict human PK from animal data, enhancing translational accuracy.
Service you may interested in
Differences between ADME and DMPK: Unpacking the Nuances
Scope and Breadth
ADME focuses on describing individual processes (e.g., how a drug is absorbed), while DMPK encompasses ADME and integrates PK, metabolism, and often toxicology to address strategic questions in drug development (e.g., "Will this compound achieve therapeutic levels in the brain?").
Focus and Objectives
ADME is descriptive, characterizing a drug's fate, whereas DMPK is predictive and prescriptive, aiming to optimize drug candidates. For instance, ADME may identify that a drug is rapidly metabolized, while DMPK uses this data to model how structural changes could extend its half-life.
Methodologies and Tools
ADME relies on in vitro assays (e.g., Caco-2 cell permeability, hepatocyte metabolism) and early in vivo studies in simple models. DMPK employs more complex tools: quantitative bioanalysis (e.g., LC-MS/MS for drug levels), PK modeling software (e.g., NONMEM), in silico predictions, and PBPK models.
Stage of Application
ADME studies typically occur in early discovery to screen compounds (e.g., eliminating those with poor solubility). DMPK continues through preclinical and clinical phases, informing decisions like starting doses in human trials or adjusting regimens for patients with hepatic impairment.
Connections Between ADME and DMPK: A Symbiotic Relationship
ADME as the Foundation for DMPK
DMPK cannot exist without ADME data. For example, knowing a drug's hepatic clearance (an ADME parameter) is essential for building a PK model to predict steady-state concentrations.
DMPK as the Strategic Application of ADME
DMPK translates ADME findings into actionable insights. Suppose ADME reveals high first-pass metabolism; DMPK may suggest an alternative route of administration (e.g., intravenous) or structural modifications to reduce metabolism.
The Iterative Feedback Loop
DMPK results can highlight gaps in ADME knowledge. If a PK model predicts unexpected toxicity, researchers may revisit ADME studies to characterize a previously overlooked metabolite. Conversely, DMPK insights can guide ADME optimization, such as designing a compound with reduced plasma protein binding to increase active drug concentration.
Case Study: Interplay in Action
A compound shows low oral bioavailability in ADME studies due to efflux by P-glycoprotein. DMPK modeling predicts that co-administering a P-glycoprotein inhibitor could enhance bioavailability, but this approach is deemed risky. Instead, chemists modify the compound to evade P-glycoprotein, and subsequent DMPK studies validate improved oral exposure.
The Strategic Importance of DMPK and ADME in Drug Discovery
Early Integration: Fail Early, Fail Cheap
Screening for ADME/DMPK liabilities (e.g., high clearance, poor CNS penetration) in lead optimization reduces the likelihood of late-stage failures. For example, identifying a compound with short half-life early allows researchers to prioritize analogs with longer half-lives.
Optimizing Drug Candidate Selection
DMPK criteria (e.g., balanced clearance, suitable volume of distribution) help rank candidates. A compound with linear PK (i.e., predictable exposure with dose) is preferable to one with nonlinear PK, which complicates dosing.
Informing Clinical Development
PK data guides dose escalation in phase I trials and predicts exposure in special populations (e.g., elderly patients with reduced renal function). Translational PK models bridge preclinical and clinical data, minimizing trial design risks.
Impact on Commercial Viability
Drugs with favorable ADME/DMPK profiles (e.g., once-daily dosing, low DDI potential) are more marketable. For instance, a drug with minimal food effect on absorption simplifies patient compliance.
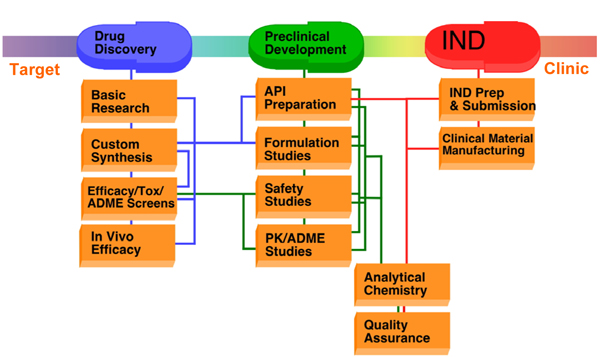 Fig.1 Stages of preclinical drug development. 1,2
Fig.1 Stages of preclinical drug development. 1,2
Future Directions in ADME and DMPK Research
Advanced Modeling and Simulation
AI and machine learning are transforming in silico ADME/DMPK predictions, enabling faster screening of large compound libraries. PBPK models are becoming more sophisticated, incorporating organ-specific cell biology and gene expression data.
Personalized Medicine
Understanding genetic variations in ADME enzymes (e.g., CYP2C19 polymorphism) and transporters will enable tailored dosing, maximizing efficacy while minimizing toxicity in individual patients.
Novel Technologies
Organ-on-a-chip models (e.g., liver or intestine chips) recapitulate human physiology better than animal models, improving translational accuracy. Microdosing—administering sub-therapeutic doses to measure PK—reduces reliance on animal studies and speeds clinical translation.
Streamlining Drug Development
High-throughput ADME assays and integrated DMPK workflows (e.g., combining in vitro metabolism and PK modeling) are accelerating candidate optimization, reducing the time from discovery to clinical trials.
Conclusion: A Holistic View for Modern Drug Development
ADME and DMPK are distinct yet inseparable in drug research. ADME provides the foundational understanding of a drug's biological fate, while DMPK applies this knowledge strategically to predict, optimize, and advance candidates. Successful drug development hinges on leveraging both: ADME to identify liabilities early, and DMPK to translate mechanistic insights into actionable strategies. As technologies like AI and organ-on-a-chip models evolve, the synergy between ADME and DMPK will only grow, driving the discovery of safer, more effective therapeutics for patients.
If you want to learn more about the transgenic mice, please refer to:
- Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
- In Vitro ADME Screening: Accelerating Drug Development
- In Vitro ADME Assays: Principles, Applications, and Protocols
- In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insight
- In Vitro ADME and In Vivo PK Studies: A Holistic Framework for Drug Evaluation
- ADME in Toxicology: Ensuring Drug Safety and Efficacy
If you are seeking detailed ADME or DMPK testing protocols for your candidate compounds, don't hesitate to connect with our expert team. At Creative Biolabs, we specialize in delivering tailored analytical solutions that streamline your research workflow and enhance efficiency. Our seasoned scientists are ready to collaborate with you, providing comprehensive strategies to optimize your candidate evaluation process. Contact us today to unlock the potential of your research and accelerate your project timeline.
References
- Steinmetz, Karen L., and Edward G. Spack. "The basics of preclinical drug development for neurodegenerative disease indications." BMC neurology 9.Suppl 1 (2009): S2. https://doi.org/10.1186/1471-2377-9-S1-S2
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
