Humanized Mice: A Revolutionary Tool for Neurological Disease Research
Humanized mouse models have emerged as indispensable tools in biomedical research, particularly for unraveling the complexities of neurological disorders. By integrating human genetic, cellular, or tissue components into mice, these models bridge the species gap, enabling scientists to mimic human pathophysiology with unprecedented accuracy. This article explores the applications, advancements, and future prospects of humanized mice in neurological disease research, highlighting their role in accelerating drug development and mechanistic understanding.
Definition and Classification of Humanized Mice
Humanized mice are genetically or surgically modified rodents that carry human functional genes, cells, tissues, or immune systems. The core advantage of these models lies in their ability to overcome species-specific differences, providing a more relevant platform for studying human diseases. There are three primary categories:
Genetically Humanized Mice: These models feature human genes replacing their murine counterparts. For example, genes like MAPT (encoding tau protein) or SNCA (encoding α-synuclein) are introduced to express human proteins associated with neurodegeneration.
Immune System Humanized Mice: Created by transplanting human peripheral blood mononuclear cells (PBMCs) or hematopoietic stem cells (HSCs) into immunodeficient mice (e.g., NOG/NSG mice), these models reconstruct a human-like immune system, crucial for studying immune-mediated neurological disorders.
Organ/Tissue Humanized Mice: Involves transplanting human brain cells (e.g., microglia) into the mouse central nervous system, allowing researchers to investigate human-specific cellular interactions in the brain.
Key technologies underpinning these models include CRISPR/Cas9 gene editing for precise genomic modification, embryonic stem (ES) cell targeting for long-fragment gene insertion, and optimized techniques to minimize off-target effects.
Research Challenges in Neurological Diseases and the Value of Humanized Models
Neurological diseases, encompassing neurodegenerative disorders (e.g., Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis), neurodevelopmental disorders (e.g., autism), and autoimmune conditions (e.g., multiple sclerosis), present significant research hurdles:
Species Barrier: Structural and functional differences between mouse and human brains, along with divergent immune responses, limit the predictive value of traditional rodent models. For instance, classic AD mouse models often fail to recapitulate widespread neuronal loss seen in humans.
Mechanistic Complexity: Diseases involve intricate gene-environment-immune interactions. Take microglia in AD, which exhibit both neuroprotective and toxic roles—a duality hard to model in mice.
Low Drug Translation Rate: Over 90% of central nervous system (CNS) drugs fail in clinical trials, underscoring the need for preclinical models that better predict human efficacy.
Humanized mice address these challenges by:
Precisely modeling human targets: For example, B6-hSNCA mice expressing human α-synuclein replicate Lewy body formation in Parkinson's disease.
Enabling immune interaction studies: Humanized immune systems allow modeling of neuroinflammation, such as CD8+T cell infiltration in multiple sclerosis.
Supporting personalized medicine: Models built with patient-derived induced pluripotent stem cells (iPSCs) facilitate tailored therapeutic development.
Service you may interested in
Applications and Breakthroughs in Key Neurological Diseases
Neurodegenerative Diseases
-
Alzheimer's Disease (AD)
B6-htau mice: With humanized MAPT genes, these mice express human tau protein isoforms, serving as a platform for screening siRNA therapies that reduce tau mRNA levels.
Microglia transplantation models: Studies have implanted human iPSC-derived microglia into mouse brains, achieving up to 80% human cell engraftment. This allows dynamic modeling of Aβ-induced neuroinflammation, fill in the gaps in traditional models.
Novel microglia models: Recent advancements enable human microglia engraftment without triggering immune rejection, providing new tools for AD mechanism research and drug delivery system development. -
Parkinson's Disease (PD) and Amyotrophic Lateral Sclerosis (ALS)
B6-hSNCA mice: Humanized for SNCA, these models support the screening of α-synuclein antibody therapies.
C9orf72 humanized models: Used to evaluate antisense oligonucleotide (ASO) treatments for ALS, validating target binding efficiency in preclinical settings.
Neuroimmune Diseases
-
Multiple Sclerosis (MS)
PBMC-humanized NOG mice: Transplantation of Epstein-Barr virus-infected human PBMCs recapitulates CD8+ T cell brain infiltration and autoimmune attacks, overcoming limitations of traditional models that lack human-virus interaction mechanisms. -
Systemic Lupus Erythematosus (SLE) with Neuroinvolvement
HSC-humanized models: Reconstructing human immune systems allows investigation of autoantibody-mediated neuronal damage mechanisms.
Neurodevelopmental Disorders
-
Autism Spectrum Disorder (ASD)
Gut-brain axis models: Transplanting ASD patient-derived gut microbiota into germ-free mice induces social deficits and repetitive behaviors, validating the therapeutic effects of prebiotics (e.g., taurine) in modulating neural excitability.
SYNGAP1/STXBP1 humanized models: These models, with human gene orthologs, facilitate preclinical testing of targeted therapies, including gene therapies.
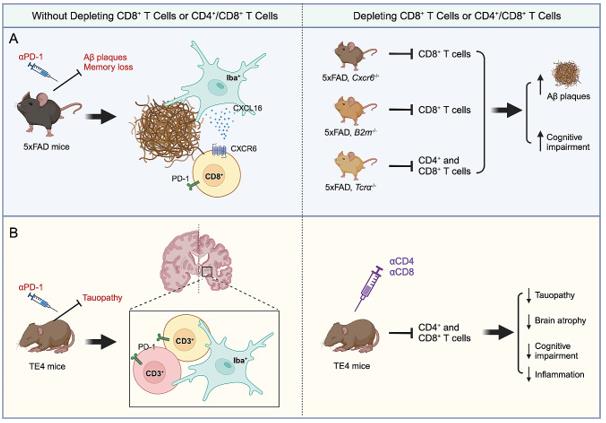 Fig.1 Two alzheimer's disease mouse models for studying t cells in AD pathogenesis.1,2
Fig.1 Two alzheimer's disease mouse models for studying t cells in AD pathogenesis.1,2
Core Role in Drug Development
Target Validation and Efficacy Evaluation
Genetic humanized models (e.g., APOE4 humanized mice) assess the impact of ApoE modulators on lipid metabolism and neuroinflammation.
Immune humanized models test the blood-brain barrier penetration of PD-1/CTLA-4 antibodies for neuro-oncological applications.
ASO and Gene Therapy Vector Screening
Humanized Duchenne muscular dystrophy (DMD) mice validate the safety and efficacy of CRISPR-based editing therapies.
Pharmacokinetic Prediction
Mice expressing human CYP450 enzymes accurately model drug metabolism in the brain, aiding in pharmacokinetic studies.
Limitations and Future Directions
Current Challenges
Variable engraftment efficiency: Human cell survival in mouse brains varies significantly (e.g., low survival in STXBP1 models).
Incomplete complexity: Models struggle to fully recapitulate human neural networks, including the blood-brain barrier and glial cell interactions.
Ethical considerations: High-degree humanization may raise animal welfare concerns.
Technological Frontiers
- Multigene humanization: Advanced techniques enable megabase-scale gene fragment insertion, moving toward whole-genome humanization.
- Organoid transplantation: Integrating human brain organoids with mouse vascular systems enhances pathological simulation.
- AI integration: Combining big behavioral data (e.g., ASD social interaction tests) with AI improves phenotypic analysis.
Humanized mice, through genetic, immune, and tissue humanization strategies, have become central to neurological disease research. Their ability to mimic human-specific pathology, immune responses, and genetic backgrounds makes them irreplaceable for dissecting neurodegenerative mechanisms, modeling immune microenvironments, and advancing personalized medicine. Future efforts should focus on optimizing engraftment efficiency, developing multi-system integrated models, and refining ethical framework steps that will accelerate the translation of neuroscientific discoveries from bench to bedside. As technology evolves, humanized mice will continue to drive breakthroughs in understanding and treating some of the most challenging neurological disorders of our time.
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- The Role of Humanized Mice in Studying Human Immunity and Disease
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
References
- Hu, Dan, and Howard L. Weiner. "Unraveling the dual nature of brain CD8+ T cells in Alzheimer's disease." Molecular Neurodegeneration 19.1 (2024): 16. https://doi.org/10.1186/s13024-024-00706-y
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
