ADME in Toxicology: Ensuring Drug Safety & Efficacy
Introduction to ADME in Toxicology
In the realm of toxicology and drug development, the acronym ADME—standing for Absorption, Distribution, Metabolism, and Excretion—represents a fundamental framework for understanding how substances interact with biological systems. This framework has evolved to ADME-T (with "T" denoting Toxicity), emphasizing the critical role of these processes in evaluating and mitigating potential toxic effects of drugs and chemicals.
ADME studies are indispensable for ensuring drug safety. By elucidating how a compound is absorbed into the body, distributed to various tissues, metabolized, and excreted, researchers can predict and prevent toxicity risks. These insights not only safeguard patient well-being but also enhance drug efficacy by optimizing pharmacokinetic profiles. Failing to understand ADME-T dynamics can lead to severe consequences, from therapeutic failure to life-threatening adverse reactions, underscoring the need for rigorous ADME-toxicology assessments throughout the drug development pipeline.
Core Concepts of ADME-Toxicology
Absorption: Entry into the Biological System
Absorption refers to the process by which a substance enters the circulatory system from its site of administration. Key mechanisms include passive diffusion, where molecules move across cell membranes from high to low concentration, and active transport, which requires energy and specific transporters. Factors such as solubility, pH, and chemical stability significantly influence absorption efficiency. For instance, poor water solubility can hinder gastrointestinal absorption, while pH-dependent ionization affects a compound's ability to cross lipid membranes.
Toxicological risks arise when absorption is either inadequate or excessive. Inadequate absorption may lead to insufficient therapeutic exposure, whereas uncontrolled absorption can result in systemic toxicity. For example, certain protein-based therapeutics may undergo degradation in the gastrointestinal tract, reducing their efficacy and potentially causing unintended local irritation.
Distribution: Spreading Throughout the Body
Distribution involves the transport of a compound throughout the body via blood flow, influenced by factors such as protein binding and physiological barriers. Plasma proteins, particularly albumin, can bind to drugs, affecting their free concentration and availability to target tissues. Barriers like the blood-brain barrier (BBB) and placental barrier restrict access to specific organs, protecting them from potentially harmful substances.
Toxicity can emerge when compounds accumulate in non-target tissues. Heavy metals, for instance, may bind to fatty tissues, leading to prolonged exposure and cumulative damage. Similarly, compounds that cross the BBB can affect central nervous system function, potentially causing neurotoxicity. Understanding distribution patterns is crucial for predicting off-target effects and organ-specific toxicity.
Metabolism: Biotransformation and Bioactivation
Metabolism, primarily driven by liver enzymes such as the CYP450 family, converts compounds into more water-soluble forms for excretion. This process can either detoxify or activate substances. Prodrugs, for example, rely on metabolic activation to exert their therapeutic effects, but this can also lead to the formation of toxic metabolites. A classic example is paracetamol (acetaminophen), which, when metabolized in the liver, can produce the toxic intermediate N-acetyl-p-benzoquinone imine (NAPQI), causing hepatotoxicity at high doses.
Species-specific metabolic differences pose significant challenges in toxicity prediction. For instance, cats lack an efficient glucuronidation pathway, making them more susceptible to toxicity from compounds that rely on this conjugation reaction for detoxification, highlighting the need for careful consideration of species differences in preclinical studies.
Excretion: Eliminating Compounds and Metabolites
Excretion is the process by which compounds and their metabolites are removed from the body, primarily via renal, biliary, and respiratory routes. Renal excretion depends on glomerular filtration, tubular reabsorption, and secretion, while biliary excretion involves transport into bile and subsequent elimination in feces. Impaired excretion, such as in kidney or liver disease, can prolong drug half-life, increasing the risk of toxicity.
Metabolite retention can also lead to adverse effects. For example, the anticancer drug cisplatin is excreted primarily via the kidneys, and its accumulation in renal tubular cells can cause nephrotoxicity, underscoring the importance of understanding excretion kinetics to mitigate organ-specific toxicity.
Service you may interested in
ADME-Driven Toxicity Mechanisms
Case Studies: Unraveling Toxic Pathways
Metabolic activation plays a pivotal role in many drug-induced toxicities. As seen with paracetamol, normal metabolic processes can generate harmful byproducts when detoxification pathways are overwhelmed. In therapeutic doses, NAPQI is efficiently detoxified by glutathione, but overdose depletes glutathione stores, leading to liver damage.
Distribution failures can also have severe consequences. Some chemotherapy agents, for instance, may cross the placental barrier, exposing the fetus to cytotoxic effects. This highlights the need for rigorous assessment of a drug's distribution profile, especially in vulnerable populations.
Regulatory Perspectives
Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines for ADME-related toxicity assessments. These guidelines mandate comprehensive evaluation of a drug's absorption, distribution, metabolism, and excretion profiles to ensure safety before human trials, emphasizing the integration of ADME-T data throughout the drug development lifecycle.
In Vitro Toxicology Testing in ADME Studies
Key Methodologies
In vitro assays have become indispensable for ADME-toxicology evaluations:
- Cytotoxicity Assays: Techniques like the Neutral Red Uptake (NRU) assay, MTT assay, and Alamar Blue assay measure cell viability, providing insights into a compound's direct toxic effects on cells.
- Genotoxicity Tests: The Ames test, Comet assay, and micronucleus test assess a compound's potential to damage DNA, indicating genotoxic risks.
- Advanced Models: 3D cell cultures and organ-on-a-chip systems mimic human tissue physiology more accurately, enabling better prediction of in vivo ADME-T responses.
Applications in Drug Development
These models are used to predict intestinal absorption (e.g., Caco-2 cell models for evaluating intestinal permeability) and hepatic metabolism (e.g., hepatocyte cultures for assessing metabolic stability). High-throughput screening using these assays allows researchers to identify and eliminate toxic candidates early, saving time and resources in drug development.
Advantages Over In Vivo Testing
In vitro methods offer significant benefits, including cost-efficiency, reduced ethical concerns, and scalability. They enable rapid testing of large compound libraries and can be standardized to ensure reproducibility, making them invaluable tools in early-stage toxicity screening.
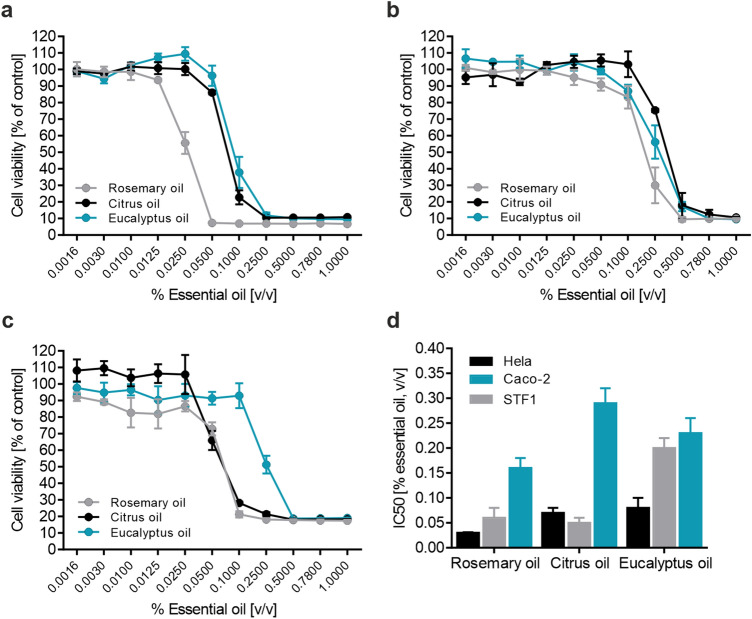 Fig.1 Evaluating EO cytotoxicity in human cell lines. 1,2
Fig.1 Evaluating EO cytotoxicity in human cell lines. 1,2
Emerging Trends in ADME-Related Toxicology Research
In Silico Innovations
Computational approaches are revolutionizing ADME-T prediction:
- QSAR Models: Quantitative Structure-Activity Relationship models correlate chemical structure with biological activity, aiding in toxicity prediction.
- Machine Learning: Advanced algorithms analyze vast datasets to predict ADME-T profiles, even for novel compounds. Virtual screening of natural products, such as phytochemicals from garlic, using these tools helps identify candidates with favorable ADME properties.
Omics Integration
Integrating genomics, transcriptomics, proteomics, and metabolomics enhances toxicity assessment:
- Genomic analyses identify genetic polymorphisms, such as variations in the CYP2D6 enzyme, that influence drug metabolism and toxicity susceptibility.
- Metabolomics profiles can identify unique biomarkers of toxicity, enabling earlier detection of adverse effects.
Personalized Medicine
Pharmacogenomics is driving personalized ADME studies, tailoring drug development to individual genetic profiles. This approach optimizes dosing regimens and predicts toxicity risks based on an individual's metabolic capacity, moving toward more precise and safer therapeutic strategies.
Case Studies: ADME in Drug Safety Successes and Failures
Success Story: Ibuprofen's Toxicity Mitigation
The non-steroidal anti-inflammatory drug (NSAID) ibuprofen exemplifies successful ADME optimization. By modifying its metabolic pathways, researchers reduced gastrointestinal toxicity while maintaining anti-inflammatory efficacy. Understanding how ibuprofen is metabolized and distributed allowed for formulation improvements that minimized off-target effects.
Failure Analysis: Terfenadine's Withdrawal
The antihistamine terfenadine was withdrawn from the market due to cardiac toxicity caused by interactions with CYP3A4 enzymes. When co-administered with drugs that inhibit CYP3A4, terfenadine accumulated, leading to QT interval prolongation and potentially fatal arrhythmias. This case underscores the critical importance of evaluating drug-drug interactions and metabolic pathways during development.
Challenges and Future Directions
Current Limitations
Despite advancements, predicting human-specific ADME-T outcomes from in vitro and in silico data remains a challenge. Species differences, complex physiological interactions, and individual genetic variations make it difficult to fully replicate human responses in preclinical models.
Innovations on the Horizon
- AI-Driven Modeling: Artificial intelligence holds promise for improving predictions, especially for rare diseases where limited data exist.
- Microphysiological Systems (MPS): These multi-organ chip systems mimic human physiology more accurately, enabling better assessment of systemic toxicity and organ-organ interactions.
ADME-T toxicology is central to ensuring drug safety and efficacy. By unraveling how compounds are absorbed, distributed, metabolized, excreted, and how these processes influence toxicity, researchers can design safer therapeutics. Integrating in vitro, in silico, and omics approaches, along with emerging technologies like MPS and AI, will further enhance our ability to predict and mitigate toxicity risks. As the field evolves, a comprehensive understanding of ADME-T dynamics will remain essential for advancing drug development and protecting public health.
If you want to learn more about the transgenic mice, please refer to:
- Comprehensive Guide to In Vitro ADME Studies in Drug Discovery
- In Vitro ADME Screening: Accelerating Drug Development
- In Vitro ADME Assays: Principles, Applications, and Protocols
- Differences and Connections Between ADME and DMPK in Drug Research
- In Vitro ADME Profiling Services: Accelerating Drug Development Through Predictive Insight
- In Vitro ADME and In Vivo PK Studies: A Holistic Framework for Drug Evaluation
References
- Lanzerstorfer, Peter, et al. "Acute, reproductive, and developmental toxicity of essential oils assessed with alternative in vitro and in vivo systems." Archives of Toxicology 95.2 (2021): 673-691. https://doi.org/10.1007/s00204-020-02945-6
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
