The Role of Humanized Mice in Studying Human Immunity & Disease
Defining Humanized Mice and Their Core Value
Humanized mouse models represent a groundbreaking advancement in biomedical research, created by transplanting human immune cells or tissues into immunodeficient mice to reconstruct functional human immune systems. The core methodologies involve transplanting human hematopoietic stem cells (HSCs), peripheral blood mononuclear cells (PBMCs), thymic tissue, or splenic/lymph node fragments. This technology bridges the gap between traditional animal models and human physiology, offering irreplaceable advantages: it mimics the complexity of the human immune system, circumvents ethical restrictions on human experiments, and overcomes the natural rejection of human cells by wild-type mice or the functional immune deficiency of conventional immunodeficient strains.
Technical Evolution and Classification of Humanized Mouse Models
Milestones in Model Development
Early iterations, such as nude mice and SCID mice, faced limitations like low transplantation success rates and radiation sensitivity. The pivotal breakthrough came in the 2000s with the development of highly immunodeficient strains (e.g., BRG, NOG, NSG), which significantly improved engraftment efficiency and enabled long-term human immune cell reconstitution.
Comparison of Three Mainstream Models
- Hu-PBMC Model: Formed by injecting human PBMCs into immunodeficient mice, this model offers low cost and operational simplicity, with detectable human CD3+ T cells within one week. However, it carries a high risk of graft-versus-host disease (GvHD), limiting its use to short-term studies.
- Hu-HSC Model: By transplanting human CD34+ HSCs, this model achieves long-term, multi-lineage immune cell reconstitution with minimal GvHD. Its key limitation is that T cells mature in the mouse thymus, lacking human-specific cytokines.
- Hu-BLT Model: Combining human fetal liver/thymus tissue with CD34+ HSCs, this model recapitulates the most comprehensive adaptive immune responses (e.g., HLA-restricted T cells). Yet, it suffers from high GvHD risk, complex surgical requirements, and high costs.
Technical Optimization Directions
- Cytokine engineering: Expressing human IL-15 has been shown to enhance natural killer (NK) cell proportions, boosting anti-tumor and antiviral research capabilities.
- Second-generation models: Introducing human Flt3 ligand genes helps reconstruct more complete NK cell, dendritic cell, and monocyte systems.
Service you may interested in
Cancer Immunotherapy: Drug Screening to Personalized Medicine
Preclinical Validation of Immune Checkpoint Inhibitors (ICIs)
Humanized mice (e.g., NSG-derived models) reconstitute human T cells, B cells, and myeloid cells, enabling the simulation of physiological interactions at checkpoints like PD-1/PD-L1 and CTLA-4. For instance, PD-1 blocking antibodies have demonstrated significant tumor growth inhibition in humanized models of gastric and non-small cell lung cancer, with efficacy highly consistent with clinical responses in patients. Combinatorial therapies have also shown promise: CTLA-4 inhibitors combined with radiotherapy activate tumor-infiltrating lymphocytes (TILs) in triple-negative breast cancer models, extending survival, while bispecific antibodies (e.g., targeting CD3×HER2) clear metastatic foci in ovarian cancer by engaging T and NK cells.
Cell Therapy Platforms: CAR-T and NK Cell Optimization
- CAR-T for solid tumors: In prostate cancer models, PSCA-targeted CAR-T cells eliminated 90% of tumor cells, while anti-CD123 CAR-T cells cleared leukemia cells in AML models. HLA-A2.1 transgenic models enhance clinical predictability by ensuring HLA-restricted tumor antigen recognition.
- NK cell therapy innovations: Engineered models expressing human IL-15 boost NK cell proportions fivefold, enhancing antibody-dependent cellular cytotoxicity (ADCC). CD19-targeted CAR-NK cells have achieved sustained remission in lymphoma models without cytokine storm risks.
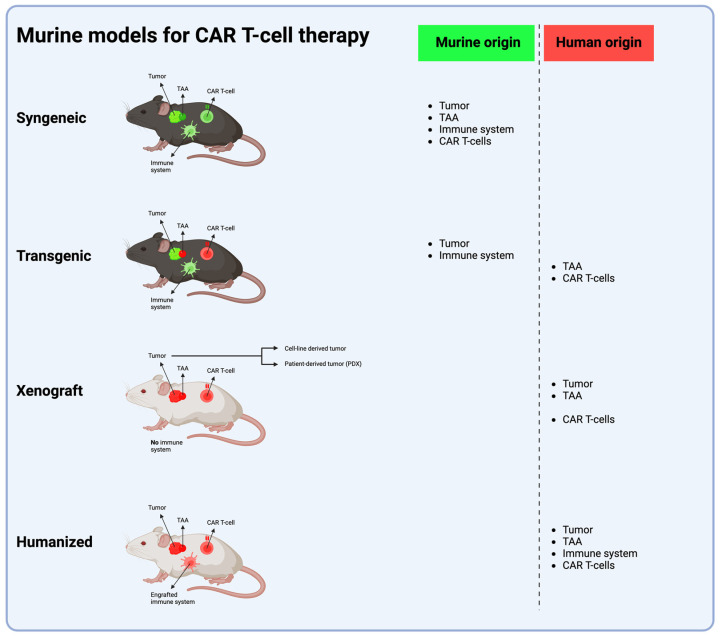 Fig.1 The four primary murine models for CAR T-cell therapy development.1,2
Fig.1 The four primary murine models for CAR T-cell therapy development.1,2
Personalized Medicine and Biomarker Discovery
PDX (patient-derived xenograft) models combined with autologous PBMC transplantation predict individual responses to PD-1 inhibitors. In melanoma models, PD-1 expression levels in TILs correlate positively with treatment efficacy.
HIV Research: Latency Mechanisms to Cure Strategies
Viral Latency Reservoirs and Clearance Mechanisms
BLT models are indispensable for modeling HIV mucosal transmission (vaginal/rectal) and revealing DC-SIGN+ dendritic cell-mediated initial infection. Latently infected cells reactivate viral replication after antiretroviral therapy (ART) discontinuation, mirroring human "cure-relapse" dynamics. Viral outgrowth assays (VOA) quantify latent cells with a sensitivity of 1 in 1 million.
Gene and Immunotherapy Breakthroughs
- Gene editing: CCR5-knockout CD34+ HSCs generate HIV-resistant T cells, while CRISPR targeting of proviral DNA reduces latency reservoirs in BLT models.
- Immune checkpoint interventions: PD-1 antibodies reverse CD8+ T cell exhaustion, lowering viral load, and LAG-3 antibodies combined with ART clear latent reservoirs.
Vaccine and Prevention Strategy Evaluation
Vaginal tenofovir gel blocks HIV transmission in BLT models, supporting pre-exposure prophylaxis (PrEP) clinical design. DNA vaccines inducing neutralizing antibodies in Zika virus-humanized mice provide a paradigm for HIV vaccine development.
Liver Diseases: From Hepatitis Modeling to Metabolic Research
Viral Hepatitis Mechanisms and Therapy
Liver-chimeric models support the complete life cycle of HBV/HCV/HEV, demonstrating that HBV cccDNA persistence drives chronic infection. Therapeutic validation includes RNaseH inhibitors reducing HBV DNA by 99% without hepatotoxicity and pegylated interferon α-2a hydrogel formulations suppressing HCV replication.
Liver Immunopathology and Fibrosis Research
Dual-chimeric models (e.g., AFC8) combine humanized liver and immune systems, revealing that HBV-specific CD8+ T cells drive liver injury. TGF-β-targeted monoclonal antibodies mitigate liver fibrosis, reducing collagen deposition by 50%.
Drug Metabolism and Toxicity Prediction
Humanized liver mice (e.g., PXB models) accurately predict mitochondrial hepatotoxicity of nucleoside analogs like FIAU. High-fat diets induce non-alcoholic steatohepatitis (NASH) in these models, enabling evaluation of PPARδ agonist efficacy.
Frontiers and Challenges
Technological Innovation Directions
- Multi-tissue humanization: Liver-immune dual-chimeric models (e.g., AFC8) facilitate studies of chronic hepatitis immune responses.
- Gene editing acceleration: CRISPR-mediated knock in of human CD4/CCR5 genes creates more HIV-susceptible mouse models.
Existing Limitations and Countermeasures
| Challenge | Strategy |
|---|---|
| GvHD | Use low-GvHD strains like SRG/TKO-BLT |
| Human cytokine deficiency | Transgenically express human IL-15, M-CSF, etc. |
| Inadequate myeloid cell reconstitution | Combine HSC transplantation with cytokine engineering |
Standardization and Commercialization
Commercial providers offer ready-to-use models (e.g., NSG-HIS variants, huHSC-NKG) to shorten research cycles, while institutions like the NIH fund over 500 projects to advance model optimization and clinical translation.
Humanized mouse models have become indispensable platforms for interdisciplinary research in immunology, oncology, and infectious diseases. Their value lies in:
- Preclinical predictability: Bridging the translational gap between traditional models and human trials, especially for personalized medicine.
- Interdisciplinary integration: Enabling breakthroughs from HIV latency eradication to NASH drug development via humanized microenvironments.
- Future directions: Gene editing and multi-tissue engineering will drive the creation of comprehensive "human immune-liver-tumor" tripartite models, further elevating their translational impact.
Accelerate Your Disease Research with Custom Humanized Mouse Tumor Models
At Creative Biolabs, we specialize in developing humanized mouse tumor models for a wide range of diseases. We understand that effective research requires precision. That's why we're committed to building the most suitable model tailored to your specific research characteristics.
Ready to discuss your project and create the ideal model for your disease research? Contact us today to get started!
If you want to learn more about the humanized mice, please refer to:
- What Are Humanized Mice? A Comprehensive Overview of Their Definition and Applications
- How to Create Humanized Mice: A Step-by-Step Guide for Researchers
- Humanized Mice in Immuno-Oncology Research: Progress and Challenges
- Humanized Mice Models: From COVID-19 to Cancer – Key Applications
- Humanized Mice for Antibody Production and Disease Modeling
- Humanized Mice in Vaccine Research: From Concept to Clinical Trials
- Humanized Mice in Breast Cancer Research: Modeling Tumor Progression and Treatment
- The Core Applications of Humanized Mice in Prostate Cancer Research
- Humanized Mice: A Revolutionary Tool for Neurological Disease Research
References
- Vandecandelaere, Gust, et al. "Pre-Clinical Models for CAR T-Cell Therapy for Glioma." Cells 13.17 (2024): 1480. https://doi.org/10.3390/cells13171480
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
