Advances in Genetic Engineering: CRISPR & BAC Technologies in Transgenic Mice
As a core tool for biomedical research, transgenic mice play an irreplaceable role in the analysis of disease mechanisms, drug screening, and gene function research. Although traditional technologies such as pronuclear injection and homologous recombination have promoted early research, their defects such as unstable expression and low efficiency (usually the first mouse generation rate is less than 10%) caused by random insertion have limited the construction of complex models. With the breakthrough of genome editing technology, CRISPR-Cas9 and bacterial artificial chromosome (BAC) technology have gradually become the dual engines for precise manipulation of the mouse genome. CRISPR has revolutionized gene knockout and point mutation strategies with its efficient targeted editing capabilities; and BAC technology, with its advantage of carrying large gene fragments, provides a new path for the functional study of complex regulatory elements.
This article aims to systematically analyze the principles, operation procedures and applicable scenarios of CRISPR and BAC technologies, compare the differences between the two in editing accuracy, efficiency and application potential, and explore the role of their collaborative innovation in promoting precision medicine. By analyzing the latest research cases and technological advances, it provides scientific references for researchers to choose suitable Genome Editing Mice construction strategies, and helps optimize and standardize Transgenic Mouse Technology.
CRISPR-Cas9 technology: a powerful tool for precise gene editing
Basic principles and operation procedures
The CRISPR-Cas9 system forms a complex with the Cas9 nuclease through single-stranded guide RNA (sgRNA), targets specific genomic sequences and induces double-strand breaks (DSBs), and then relies on non-homologous end joining (NHEJ) or homology-directed repair (HDR) mechanisms to achieve gene editing. Its core operations include: sgRNA design and in vitro transcription, co-injection of Cas9 mRNA or protein and sgRNA into the pronucleus of fertilized mouse eggs, and embryo transplantation into the uterus of pseudo-pregnant female mice. After verification by PCR and sequencing, homozygous mutants can be obtained through rapid breeding (only 1-2 generations), and the efficiency of biallelic gene knockout can be as high as 80%.
Building strategic and technical advantages
The flexibility of CRISPR supports a variety of editing modes:
Gene knock-in: inserting exogenous genes (such as fluorescent reporter genes) through HDR templates;
Conditional editing: Combined with the Cre-loxP system, tissue-specific gene expression regulation can be achieved;
Multi-gene editing: By co-transfecting multiple sgRNAs, simultaneous knockout of T cell checkpoint genes such as PD-1/CTLA-4 can be achieved.
Its technical advantages are reflected in:
- High efficiency: Compared with traditional ES cell targeting, the cycle is shortened from 18 months to 6 months;
- Low cost: The cost of a single project can be controlled within US$5,000;
- Precision: Single-base editing tools (such as BE4max) can correct pathogenic point mutations.
However, CRISPR still faces challenges such as off-target effects and low efficiency of large knock-in fragments (>5kb). Recent advances such as Prime Editing and the application of epigenetic editors (dCas9-p300) have further expanded its potential in epigenetic regulation and gene activation/repression research.
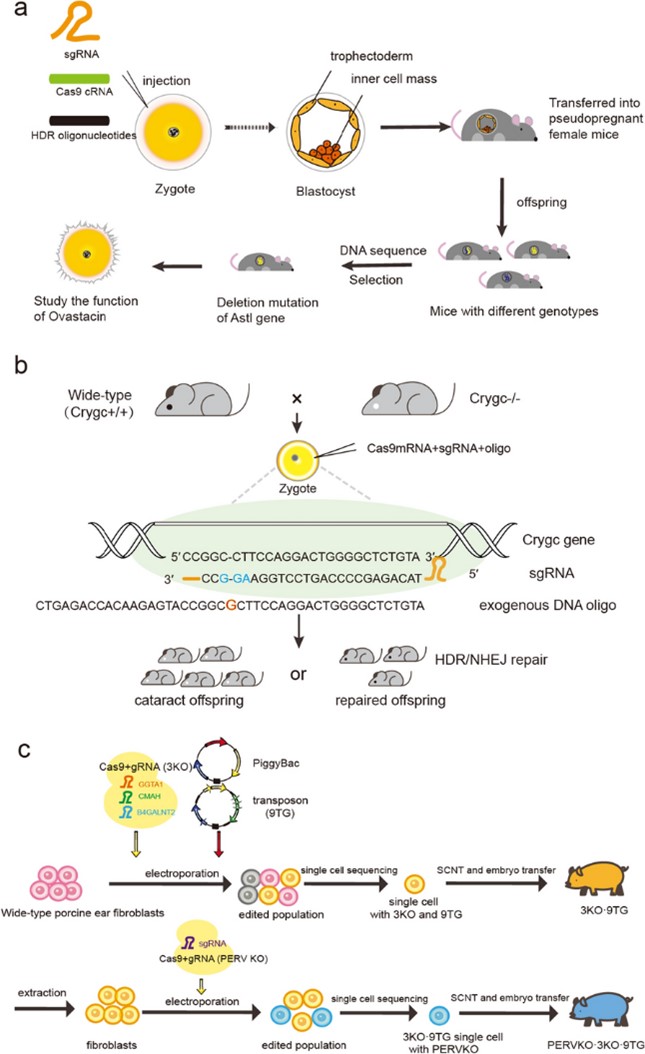 Fig. 1 Schematic diagram of mammalian CRISPR/Cas9 gene editing.1,2
Fig. 1 Schematic diagram of mammalian CRISPR/Cas9 gene editing.1,2
BAC technology: the cornerstone of large-fragment gene manipulation
Technical principles and operation procedures
Bacterial artificial chromosomes (BAC) can carry up to 300kb of genomic DNA and retain the regulatory elements of genes (such as promoters and enhancers) intact, thereby achieving physiological levels of spatiotemporal specific expression in transgenic mice. The typical process includes: inserting the target fragment into the BAC vector through homologous recombination, linearizing and microinjecting into the pronucleus of fertilized eggs, screening founder mice, and verifying the integration site by Southern Blot or fluorescent labeling. For example, the Adipoq-CreERT2 BAC-TG model uses an adipocyte-specific promoter to achieve precise expression of the inducible Cre recombinase.
Application scenarios and limitations
The core advantages of BAC technology are:
- Complex gene cluster research: For example, the Huntington's disease BACHD model carries the full-length human HTT gene (containing 67 CAG repeats), accurately simulating neurodegenerative lesions;
- Construction of humanized model: HLA-A11/hTAP-LMP mice improve the reliability of vaccine immunogenicity evaluation by integrating human MHC genes.
However, its limitations cannot be ignored: the generation rate of first-time mice is usually less than 5%, and random integration may lead to expression silencing. To overcome this defect, researchers have developed a Cre-loxP-mediated site-specific integration strategy, combined with CRISPR-induced site-specific cleavage, which can increase the BAC insertion efficiency by 3 times.
The coordinated application of the two can break through the bottleneck of a single technology.
Table 1 CRISPR vs BAC: Technology Comparison and Collaborative Innovation
| CRISPR-Cas9 | BAC Technology | |
|---|---|---|
| Editing Precision | Single-base to small-fragment editing | Precise integration of large fragments (>100kb) |
| Efficiency | High (homozygous mutation rate up to 80%) | Low (founder mouse generation rate <5%) |
| Cost and Timeline | Low cost ($5,000) and short timeline (6 months) | High cost (complex restructuring required, $15,000+/12 months) and long timeline |
| Application Scenarios | Gene knockout, point mutation, conditional models | Complex regulatory element studies, humanized models |
| Synergistic Potential | Using BAC as an HDR template for large-fragment knock-in | Combining CRISPR for site-specific integration to avoid random insertion issues |
Service you may interested in
Ethical Controversy and Future Prospects
Although gene editing technology has promoted the construction of personalized disease models, animal welfare (such as embryonic lethality caused by gene editing) and ecological risks (transgenic escape) still need to be strictly regulated. In the future, the combination of CRISPR-BAC joint strategy, single-cell embryo editing and organoid technology is expected to achieve a breakthrough in the entire chain of "precise editing-efficient verification-clinical transformation" and accelerate the transformation process from basic research to new drug development.
The complementarity and synergy of CRISPR and BAC technologies are reshaping the construction paradigm of transgenic mouse models. With the popularization of standardized databases (such as the Ensembl BAC library) and automated design tools (such as CRISPOR), genetic engineering technology will serve precision medicine more efficiently and provide a powerful engine for the analysis of disease mechanisms and the development of treatment strategies.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Are you facing challenges with BAC or CRISPR-based mouse model construction? Connect with Creative Biolabs' expert team today for comprehensive support and to discuss a tailored strategy that meets your specific research needs. We're here to help you overcome hurdles and achieve your model development goals.
References
- Zhang, Yi-ran, Tai-lang Yin, and Li-quan Zhou. "CRISPR/Cas9 technology: applications in oocytes and early embryos." Journal of Translational Medicine 21.1 (2023): 746. https://doi.org/10.1186/s12967-023-04610-9
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
