Transgenic Reporter Mice: Tools for Visualizing Gene Expression
In the vast field of life sciences, the dynamic regulation of gene expression has always been one of the core issues. Scientists have long been committed to answering a key question: How to track the activity trajectory of specific genes in real time and accurately in complex organisms? This not only involves the spatiotemporal specificity of gene expression, but also the core mechanisms of a series of basic and applied research such as cell differentiation and disease occurrence. As the basic unit of life activities, the expression pattern of genes directly determines the functional state of cells, and abnormal gene expression is often the initial cause of major diseases such as cancer and neurodegenerative diseases. Therefore, technical tools for efficient visualization of gene expression have become the key to unlocking the mysteries of life.
The emergence of transgenic reporter gene mice provides a breakthrough solution to this challenge. This type of model organism constructed through genetic engineering technology can convert the expression of target genes into detectable signals (such as fluorescence and bioluminescence), thereby realizing real-time tracking of gene activity. The core principle is to connect the reporter gene (such as green fluorescent protein gene, luciferase gene) to the regulatory element of the target gene so that the expression of the reporter gene is strictly regulated by the target gene. When the target gene starts to express, the reporter gene is synchronously activated to generate a signal that can be detected by the instrument, and then the dynamic process of gene activity is intuitively presented through imaging technology. This technology not only revolutionizes the means of traditional gene expression research, but also shows great potential in the fields of disease model construction and drug development.
Core concepts and working principles of transgenic reporter mice
Basic Concepts
- Transgenic refers to the technology of artificially introducing foreign genes into the genome of the recipient organism and making it stable. This process usually uses vectors (such as lentivirus, plasmid) or microinjection technology to integrate the target gene fragment into the chromosome of the host cell.
- Reporter Gene is a type of gene that encodes detectable products, and its expression products can be visually observed through physical, chemical or biological methods. Common reporter genes include green fluorescent protein (GFP), luciferase, β-galactosidase (LacZ), etc. Their products correspond to fluorescent signals, bioluminescent signals or enzymatic color development reactions.
- The transgenic reporter gene mouse is a mouse model that can stably express the reporter gene, which is obtained by connecting the reporter gene to the regulatory sequence of the target gene (such as the promoter and enhancer) through transgenic technology and then introducing it into mouse embryonic stem cells or fertilized eggs. Its core design is that the expression of the reporter gene is strictly controlled by the regulatory elements of the target gene, thus becoming an "indicator" of the expression of the target gene.
Working Principle
The working mechanism of transgenic reporter gene mice is based on the principle of "co-expression": when the regulatory elements of the target gene are activated (such as in a specific tissue, cell type or external stimulus), the reporter gene connected to it starts transcription and translation synchronously to produce detectable signal molecules. For example, if the promoter of the target gene is neuron-specific, then the reporter gene will only be expressed in neurons, thereby marking this type of cell.
The core of signal detection lies in the characteristics of the reporter gene product: GFP-like proteins do not require exogenous substrates and spontaneously fluoresce under excitation light of a specific wavelength; Luciferase requires a substrate (such as fluorescein) to participate in the reaction and releases photons through enzymatic oxidation; LacZ can catalyze X-Gal color development to generate a blue product. The intensity of these signals is positively correlated with the expression level of the target gene, so it can quantitatively reflect the dynamic changes in gene activity. Through equipment such as fluorescence microscopes and in vivo imaging systems, researchers can observe the spatiotemporal patterns of gene expression in real time at the cell, tissue, and even whole animal levels.
Mainstream Technology Types: Principles, Advantages and Typical Application Scenarios
GFP transgenic mice: a "green beacon" for cell tracking
Green fluorescent protein (GFP) originated from the Victoria jellyfish. Its natural fluorescent properties emit green visible light under ultraviolet excitation. Through lentiviral vectors or embryonic microinjection technology, the GFP gene can be integrated into the mouse genome and connected to the regulatory elements of the target gene. The improved version EGFP (enhanced GFP) increases the fluorescence brightness by 30-40 times through codon optimization and reduces the folding error rate in mammalian cells, making it a more efficient reporting tool.
In tumor research, GFP-labeled host immune cells (such as T cells) are injected into immunodeficient nude mice, and combined with red fluorescent-labeled tumor cells, the recruitment and functional changes of immune cells during tumor invasion can be intuitively analyzed. In addition, GFP transgenic mice are also used in cardiovascular development research to track the dynamic behavior of endothelial cells in angiogenesis.
The advantages of GFP transgenic mice are as follows:
- Real-time dynamic monitoring: No exogenous substrate is required, and cell migration (such as the migration of neural stem cells from the ventricular zone to the cortex) and differentiation processes can be tracked directly through fluorescence imaging.
- Long-term stability: GFP protein is resistant to photobleaching and is suitable for long-term in vivo observation, such as the study of the interaction between host cells and cancer cells in the tumor microenvironment.
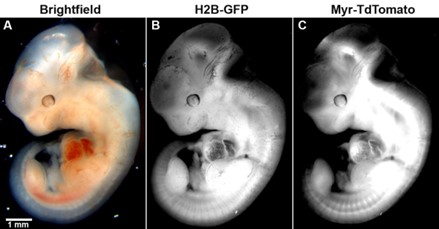 Fig. 1 Picture of Tom-2A-GFP widely expressed in transgenic embryos.1,2
Fig. 1 Picture of Tom-2A-GFP widely expressed in transgenic embryos.1,2
EGFP transgenic mice: an upgraded option for high-resolution imaging
Compared with traditional GFP, EGFP has two core advantages: first, the excitation spectrum is red-shifted to 490nm, which reduces the interference of tissue autofluorescence (such as the strong absorption of hemoglobin at short wavelengths) and significantly improves the imaging signal-to-noise ratio; second, the thermal stability is enhanced, and it can be expressed stably and for a long time at mammalian body temperature (37°C), avoiding the abnormal conformation of GFP at high temperature.
EGFP transgenic mice are usually used as follows:
- Multicolor labeling technology: EGFP can be used in combination with EYFP (yellow fluorescent protein), ECFP (cyan fluorescent protein), etc., to achieve synchronous tracking of multiple signal pathways in the same mouse through the combination of different excitation/emission wavelengths. For example, in neural circuit research, EGFP labels excitatory neurons and EYFP labels inhibitory neurons. Combined with two-photon microscopy, neural connections in specific brain regions can be analyzed.
- Imaging of controversial areas: In deep or structurally complex areas of brain tissue (such as the hippocampus and amygdala), the high brightness and anti-interference properties of EGFP support high-resolution imaging, providing a precise tool for studying the degeneration of specific neuronal subpopulations in neurodegenerative diseases.
Luciferase transgenic mice: the gold standard for in vivo quantitative analysis
Luciferase (such as firefly Luciferase) catalyzes the oxidation of substrate luciferin to generate oxyluciferin and release photons with a wavelength of 560nm. In recent years, the autonomous luminescence system (such as the bacterial Lux system) developed does not require exogenous substrates. By integrating the luciferin synthesis pathway gene, it can achieve continuous luminescence in mice, further simplifying the experimental process.
In oncology, Luciferase-labeled tumor cells are used to monitor in situ tumor growth, lymph node metastasis, and distant organ colonization (such as lung and liver metastases) in real time. In circadian rhythm research, Per2-Luc transgenic mice record liver luciferase activity to reveal the periodic expression of clock genes, providing a living model for the study of the association between sleep disorders and metabolic diseases.
Advantages of Luciferase transgenic mice:
- Ultra-high sensitivity: The detection limit can reach the single-cell level, capable of capturing extremely weak gene expression signals, and suitable for the detection and counting of circulating tumor cells (CTCs).
- Accurate quantitative analysis: There is a linear relationship between photon intensity and reporter gene expression. The in vivo imaging system can be used to dynamically monitor the spatiotemporal expression profile of inflammatory factors (such as IL-6) or the response efficiency of target genes after drug intervention.
Other reporter genes: complementary tools for diverse needs
- β-Galactosidase (LacZ): It catalyzes X-Gal to produce a blue product, which is suitable for qualitative analysis of tissue sections and is often used for localization of gene expression in early embryonic development (such as the spatiotemporal distribution of genes during neural tube formation).
- Alkaline phosphatase (AP): Combined with the substrate BCIP/NBT for color development, it can mark osteoblast differentiation in bone tissue research or be used for stem cell lineage tracing.
Service you may interested in
Technical comparison analysis: performance differences between GFP, Luciferase and EGFP
| GFP | Luciferase | EGFP | |
|---|---|---|---|
| Detection principle | Autofluorescence (excitation light required) | Substrate dependent bioluminescence | Optimized fluorescence (high brightness, low background) |
| Sensitivity | Medium (depends on the excitation light intensity) | Very high (low background noise) | High (brightness increased by 30-40 times) |
| Real-time | Instant Response | Substrate injection required (approximately 10-30 minute delay) | Instant Response |
| Applicable scenarios | Cell localization, in vitro imaging, short-term tracking | In vivo quantification, long-term dynamic monitoring, low signal detection | High-resolution tissue imaging, multi-color labeling |
| Cost and complexity | Low (no exogenous reagents required) | Medium (high substrate cost) | Medium (spectrum optimization equipment required) |
From the detection principle point of view, GFP and EGFP rely on excitation light and are suitable for rapid observation at the cell or tissue level; Luciferase achieves deep tissue penetration through bioluminescence (photons can penetrate 1-2cm thick tissue), which is suitable for overall imaging of living animals. In terms of sensitivity, Luciferase is irreplaceable in trace signal scenarios such as circulating tumor cell detection due to its extremely low background noise; and EGFP, with its brightness advantage, has become the first choice for precise positioning of subcellular structures (such as mitochondria and cell membranes). In terms of cost, the GFP system is the most economical, but Luciferase and EGFP require professional imaging equipment (such as IVIS spectral imaging system, two-photon microscope), which is suitable for research with high precision requirements.
Typical Cases in Disease Model Research
Neurodegenerative diseases: Unraveling the spatiotemporal code of neuronal degeneration
- Alzheimer's disease (AD): APP/PS1 double transgenic mice integrate the mutant genes of human amyloid precursor protein (APP) and presenilin 1 (PS1), which can simulate the deposition of amyloid β (Aβ) and neurofibrillary tangles. Combined with EGFP-labeled microglia (brain immune cells), researchers found that the abnormal activation of microglia around Aβ plaques is temporally and spatially correlated with neuronal apoptosis, providing a new perspective on the pathogenesis of AD.
- Parkinson's disease (PD): In α-synuclein (α-Syn) transgenic mice, luciferase-labeled dopaminergic neurons can monitor the degeneration process of the substantia nigra-striatum pathway in real time. Through in vivo imaging, it was found that the inflammatory factor TNF-α was significantly upregulated in the early stages of the disease, suggesting that immune intervention may become an early treatment strategy for PD.
Tumor research: decoding tumor microenvironment and metastasis mechanism
- Dual fluorescence tracing model: EGFP marks host fibroblasts and red fluorescent protein marks tumor cells. After implantation into mice, multiphoton microscopy revealed that tumor cells penetrated the basement membrane through "angiogenic mimicry", and the contractile force of matrix fibroblasts significantly promoted this process, providing a target for the development of anti-metastatic drugs.
- Dynamic monitoring of metastasis: After luciferase-labeled CTCs are injected through the tail vein, in vivo bioluminescence imaging can be used to detect cell retention in the pulmonary capillaries within 30 minutes after injection. The distribution pattern of liver and lung metastases can be clearly determined after 24 hours, revealing the key role of platelet aggregation in the initial stage of metastasis.
Infection and immunity: Uncovering the molecular landscape of host-pathogen interactions
- Viral carcinogenic mechanism: HBV transgenic mice carry the hepatitis B virus genome, and luciferase-labeled hepatocellular carcinoma (HCC) precursor cells show that the viral X protein activates the NF- κB pathway, continuously induces the expression of inflammation-related genes, and ultimately leads to malignant transformation of hepatocytes.
- Dynamic tracking of inflammation: After lipopolysaccharide (LPS) stimulation, the NF- κB activity of barrier organs such as the lungs and intestines of NF- κB -Luc transgenic mice can be observed in real time. After anti-inflammatory drug treatment, the signal intensity decreased significantly within 6 hours, providing a time basis for determining the treatment window for sepsis.
Application prospects of transgenic reporter gene mice
Technological innovation: from single labeling to multi-dimensional integration
- Innovation of autonomous luminescence system: Through gene editing technology, the luciferin synthesis pathway (such as luciferin synthase gene) is integrated into the mouse genome, completely eliminating dependence on exogenous substrates and achieving long-term, interference-free in vivo imaging, which is especially suitable for lifelong tracking of chronic diseases (such as diabetes and atherosclerosis).
- Multimodal imaging fusion: Combining fluorescence imaging (cell localization), bioluminescence (in vivo quantification) and PET (molecular targeting) technologies to build a cross-scale visualization platform. For example, EGFP marks tumor vascular endothelial cells, Luciferase monitors treatment response, and PET tracks drug distribution, achieving a one-stop analysis from cell mechanism to overall efficacy.
Precision medicine: from model building to personalized treatment
- Patient-derived customized models: Using CRISPR-Cas9 technology, the mutant genes of cancer patients (such as EGFR mutations, KRAS G12D) are inserted into the mouse reporter gene system to construct a "personalized disease model" for screening targeted drugs for the patient's specific genotype and accelerating the process of precision medicine.
- Spatiotemporal specific regulatory systems: Develop reporter gene expression modules that are light-controlled (such as light-sensitive promoters) or chemically induced (such as tetracycline regulatory systems) to achieve the "spatiotemporal switch" of gene expression. For example, in neuroscience, the reporter genes in specific brain regions are activated by light to analyze the transient neural circuit activity of memory encoding.
As a revolutionary tool for visualizing gene expression, transgenic reporter mice have overturned the observation dimension of traditional biological research. It not only allows scientists to "see" the dynamic expression of genes in the body, but also converts abstract molecular mechanisms into intuitive visual signals, providing precise technical support for disease model construction and drug development. From GFP cell tracking to in vivo quantification of Luciferase, from single gene labeling to multimodal imaging, every innovation of this technology has pushed life science research to new heights. With the rapid development of gene editing technology (such as CRISPR) and synthetic biology, the application boundaries of transgenic reporter mice will continue to expand, and they are expected to become a key bridge connecting basic research and clinical transformation in the era of precision medicine. In the future, as technology continues to mature, we may be able to truly achieve the ultimate goal of "visualizing life" - decoding the complex network of gene expression in real time at the in vivo level, and providing more precise solutions to human health dilemmas.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- How Are Transgenic Mice Created? Methods and Technologies
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
Looking for a specific reporter gene mouse model? Let's discuss your unique research needs in detail. Contact us today to explore how our expertise at Creative Biolabs can help advance your studies.
References
- Trichas, Georgios, Jo Begbie, and Shankar Srinivas. "Use of the viral 2A peptide for bicistronic expression in transgenic mice." BMC biology 6 (2008): 1-13. https://doi.org/10.1186/1741-7007-6-40
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
