How Are Transgenic Mice Created? Methods & Technologies
Introduction of Transgenic Mice
What are Transgenic Mice?
Transgenic mice are experimental animal models whose genomes are artificially modified through genetic engineering techniques. Such modifications include but are not limited to precise operations such as foreign gene introduction, endogenous gene knock-out, gene knock-in, and point mutations. In the scope of biomedical research, genetically modified mice play an extremely important role. As a core tool for studying gene functions, analyzing biological processes, and building disease models, scientists can use mouse genetic modification methods to observe genes at the level of living animals. The phenotypic effects caused by changes can further explore the basic principles of life and the mechanism of disease occurrence and development.
Purpose and Significance of Creating Transgenic Mice
- Gene function analysis: Genetically modified mice play a key role in analyzing the function of genes in the body. With the help of gene overexpression or gene knockout technology, researchers can accurately manipulate the expression level of a specific gene, allowing researchers to explore the function of the gene in many physiological processes such as growth and development processes, metabolic regulation networks, and immune response mechanisms. For example, by targeting a gene closely related to the immune system, the changes in the immune function of mice are carefully observed to clarify the exact function of the gene.
- Drug development and evaluation: In the drug research and development process, genetically modified mice serve as an important animal model and are widely used to evaluate the therapeutic effect and safety of drugs. Taking the constructed disease-specific model mice as research objects, we systematically tested the intervention effect of candidate drugs on disease phenotypes, providing key experimental data support for drugs to move from preclinical research to clinical trials.
- Applications in agriculture and biotechnology: In the field of agricultural science, genetically modified mice can be used to analyze the regulatory mechanism of genes on important traits such as animal growth performance, reproductive efficiency, and environmental adaptability, providing a theoretical basis for the formulation of livestock genetic and breeding improvement strategies. In the field of biotechnology, genetically modified mice can also be used as bioreactors to produce biological products and biomedicines with important medicinal value.
This paper aims to deeply analyze several mainstream technical paths for the construction of genetically modified mice. The traditional pronuclear microinjection method, as a classic method for the construction of early transgenic mice, will be systematically explained in this article. At the same time, with the vigorous development of gene editing technology, emerging strategies based on the CRISPR/Cas9 system will also be discussed in detail in this article. In addition, bacterial artificial chromosome (BAC) transgenic technology developed to meet the need for introduction of large DNA fragments will also become the focus of this article. Not only that, this paper will also briefly outline other auxiliary methods, and deeply analyze the advantages, limitations and application categories of different technical routes, providing theoretical basis and practical guidance for scientific researchers to reasonably choose technical solutions in practical applications.
Traditional Method: Pronuclear Microinjection
Principle of Pronuclear Microinjection
Microinjection of egg pronucleus is the direct injection of foreign DNA into the male or female pronucleus of a fertilized mouse egg. During fertilization in mice, sperm enter the egg cell and form male pronuclei and female pronuclei. At this time, the foreign DNA solution is injected into the pronucleus using microinjection technology, and the foreign DNA will be randomly integrated into the host genome. This integration is random, can occur anywhere in the genome, and the number of integrated copies is difficult to control.
Steps of Pronuclear Microinjection
-
Preparation of Donor DNA
The target gene is first cloned and inserted into an appropriate vector to construct a recombinant plasmid. Enzymatic digestion and purification steps are then used to remove the vector backbone, yielding a purified DNA fragment. The final DNA must meet specific concentration and purity standards suitable for microinjection. -
Superovulation and Collection of Fertilized Eggs
Female mice are subjected to superovulation by injecting gonadotropins to stimulate the ovaries to produce multiple oocytes. After mating, fertilized eggs are collected from the oviducts. -
Detailed Description of the Microinjection Process
Under a microscope, a microinjection needle is used to aspirate a precise amount of exogenous DNA solution and inject it into the pronucleus of the fertilized egg. This procedure requires exceptional skill and experience to avoid damaging the oocyte. -
Culture and Embryo Transfer
Injected fertilized eggs are cultured in a suitable medium for a short period to monitor their development. Embryos that develop properly are selected and transferred into the uterus of pseudopregnant female mice for further development. -
Screening and Identification of Offspring
After birth, the mice are screened using molecular biology techniques such as PCR and Southern blot to determine whether the foreign DNA has been successfully integrated into the genome, thus identifying positive transgenic mice.
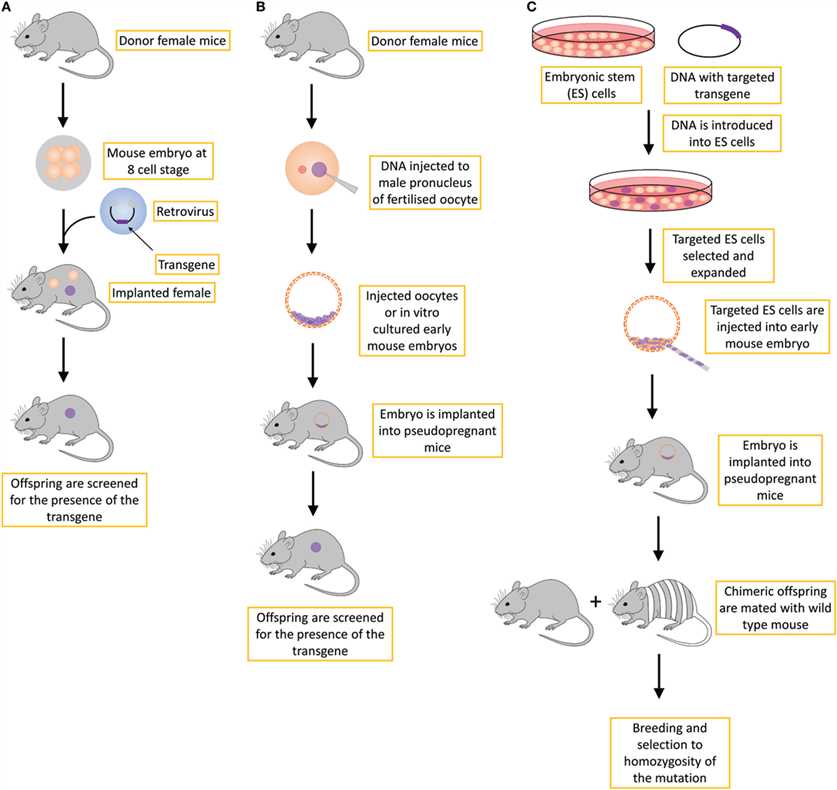 Fig.1 Different strategies for creating genetically modified mice. 1,2
Fig.1 Different strategies for creating genetically modified mice. 1,2
Advantages and Limitations of Pronuclear Microinjection
The microinjection method can directly inject foreign DNA into the pronucleus of a mouse fertilized egg. The operation is relatively simple and has little restriction on the size of the foreign DNA fragment. However, in this method, foreign DNA is randomly integrated into the host genome, which may cause insertion mutations and interfere with the normal function of the host gene. In addition, integration sites and copy numbers are difficult to accurately control, resulting in unstable expression of foreign genes and adversely affecting the repeatability and reliability of experimental results.
Emerging Technology: CRISPR/Cas9 System
Principle of CRISPR/Cas9 Gene Editing Technology
The CRISPR/Cas9 system, originally an adaptive immune mechanism in bacteria, has been engineered into a powerful and efficient gene-editing tool. Its core components include the Cas9 protein and a single-guide RNA (sgRNA). The sgRNA recognizes and binds to a specific target sequence in the genome, directing the Cas9 protein to that site to introduce a double-strand break in the DNA. The cell's own repair mechanisms then fix the break, using either non-homologous end joining (NHEJ) or homology-directed repair (HDR), enabling gene knockout, knock-in, or point mutations. This technology offers high precision, high efficiency, and multiplexing capabilities, allowing simultaneous editing of multiple genes.
Process of Creating Transgenic Mice Using CRISPR/Cas9
- Design and synthesis of sgRNA: Design specific sgRNA based on the sequence of the target gene to ensure that it can accurately identify the target site while avoiding off-target effects. Designed sgRNAs are prepared by chemical synthesis or in vitro transcription.
- Preparation of Cas9 protein or mRNA: Cas9 protein can be purified by prokaryotic expression system, or Cas9 mRNA can be prepared and synthesized by in vitro transcription.
- Various methods for introducing CRISPR/Cas9 components into fertilized eggs: Commonly used methods include microinjection and electroporation. Microinjection involves mixing sgRNA and Cas9 protein or mRNA and injecting them into the cytoplasm or pronucleus of a mouse fertilized egg; electroporation uses electrical pulses to form temporary holes in the cell membrane to allow CRISPR/Cas9 components to enter the cell.
- Evaluation of gene editing efficiency and screening of positive mice: Mice after gene editing were tested through sequencing and other methods to evaluate editing efficiency. Positive mice carrying the target gene editing were screened out and used for subsequent research.
Advantages of CRISPR/Cas9 in Transgenic Mice Creation
- Higher targeting and accuracy: The specific recognition of sgRNA allows Cas9 protein to accurately locate to the target gene locus, greatly reducing off-target effects and improving the accuracy of gene editing.
- Higher efficiency: Compared with traditional methods, the CRISPR/Cas9 system can achieve gene editing more efficiently, especially in terms of gene knockouts, with significantly improved efficiency.
- A variety of genetic modifications can be realized: it can realize a variety of operations such as gene knock-in, knockout, and point mutation to meet different research needs. For example, in the construction of disease models, genetic mutations associated with human diseases can be accurately simulated.
- Potential for simultaneous editing of multiple genes: By designing multiple sgRNAs, multiple genes can be edited simultaneously, providing a powerful tool for studying complex gene networks and polygenic diseases.
Introducing Large DNA Fragments: BAC Transgenic Technology
Principle of BAC Transgenic Technology
Bacterial artificial chromosome (BAC) vector is a cloning vector that can accommodate large fragments of DNA and has the characteristics of stability and low copy number. It can carry fragments of genomic DNA hundreds of kb long, including the entire gene and its regulatory elements. In BAC transgenic technology, BAC DNA containing the target gene or gene cluster is introduced into a mouse fertilized egg and integrated into the mouse genome. Because BAC DNA carries complete regulatory elements, it can better simulate the natural expression pattern of genes in the body.
Process of Creating BAC Transgenic Mice
- Construction of a BAC clone containing the target DNA fragment: Select a BAC clone containing the target gene from a genomic library, or construct a recombinant BAC clone through molecular biological methods to ensure that it contains the required complete gene and regulatory sequences.
- Linearization and purification of BAC DNA: Use restriction enzymes to linearize BAC DNA to remove irrelevant sequences such as the origin of replication of the vector, and then obtain high-purity linear BAC DNA through purification methods.
- Microinjection of BAC DNA into the pronucleus of a fertilized egg: Similar to microinjection of the pronucleus of an egg, the purified BAC DNA solution is injected into the pronucleus of a mouse fertilized egg to integrate it into the genome.
- Screening and identification of descendants: PCR, fluorescence in situ hybridization (FISH) and other methods were used to detect whether BAC DNA fragments were inserted into the mouse genome, and positive transgenic mice were selected.
Advantages and Applications of BAC Transgenic Technology
This technology has unique advantages in analyzing complex gene regulatory networks and can conduct in-depth research on the regulatory mechanism of the synergistic action of multiple genes. At the same time, it performs well in the field of disease model construction. It can build complex genetic disease models containing multiple genes, providing a highly realistic in vivo environment for exploring the occurrence and development of diseases, and greatly promoting the progress of related research.
Service you may interested in
Brief Introduction to Other Methods
Retrovirus-mediated gene transfer
Retroviruses have the ability to integrate their own genome into the genome of host cells. The target gene is inserted into a retroviral vector, and the virus is used to infect early mouse embryos or embryonic stem cells to achieve the introduction of foreign genes. The advantages of this method are high infection efficiency, but the capacity of the virus vector is limited and there is a risk of insertion mutation.
Lentivirus-mediated gene transfer
Lentiviruses are a type of retrovirus that can infect dividing and non-dividing cells. Compared with ordinary retroviruses, lentiviral vectors have larger capacity and more stable integration capabilities, making them suitable for introducing foreign genes into cell types that are difficult to infect, such as neurons.
Embryonic Stem (ES) Cell Gene Targeting
Although ES cell gene targeting is commonly used for gene knockouts, foreign genes can also be introduced through specific methods. First, gene targeting was carried out in ES cells, positive ES cells were screened out, and then injected into mouse blastocysts to allow them to participate in embryonic development, and finally a transgenic mouse was obtained. The advantage of this method is that it has high gene targeting efficiency, but it requires the cultivation of ES cells and the operation process is relatively complex.
Choosing the Appropriate Method
Different research objectives are adapted to different techniques. For gene overexpression and small target gene fragments, egg pronucleus microinjection or CRISPR/Cas9 system can be used; if gene knockout, knock-in, point mutation, or point mutation needs to accurately simulate human diseases, CRISPR/Cas9 system has significant advantages; BAC transgenic technology is more suitable for studying complex gene regulation, introducing large gene fragments, or requiring high integration sites and expression patterns.
From the technical difficulty and cost point of view, egg pronucleus microinjection is simple and low cost, suitable for laboratory preliminary attempt; CRISPR/Cas9 system needs to design sgRNA and prepare Cas9 components, which is slightly more difficult but controllable in cost; BAC transgenic technology is complex to operate, which requires BAC cloning and processing large DNA fragments, which is costly and suitable for specific complex research.
In terms of efficiency and accuracy, CRISPR/Cas9 system can meet the high requirements for gene editing accuracy and efficiency; egg pronucleus microinjection is relatively low in these two aspects and suitable for less demanding research.
Conclusion and Future Perspectives
As gene editing technology continues to advance, methods for creating genetically modified mice have become increasingly rich and efficient. Traditional egg pronucleus microinjection technology is mature and low-cost, but it has the problem of random integration; the CRISPR/Cas9 system has high precision and efficiency and can achieve multiple genetic modifications; BAC transgenic technology can introduce large DNA fragments and maintain gene expression patterns. These technologies continue to develop in biomedical research and will play a key role in disease model construction, gene function research, drug development and other fields, helping to create more accurate and complex genetically modified mouse models, and bringing new benefits to human disease treatment and biological research. More breakthroughs.
If you want to learn more about the transgenic mice, please refer to:
- What Are Transgenic Mice? Definition, Types, and Key Concepts
- Transgenic Mice in Cancer Research: From Tumor Models to Therapy Development
- Applications of Transgenic Mice in Disease Research and Drug Development
- Transgenic Mice vs Knockout Mice: Understanding the Differences and Research Benefits
- Humanized Transgenic Mice: Bridging Animal Models and Human Disease Studies
- Inducible and Conditional Transgenic Mice Tools for Controlled Gene Expression
- Popular Transgenic Mouse Models in Neuroscience and Immunology
- Advances in Genetic Engineering: CRISPR and BAC Technologies in Transgenic Mice
- Transgenic Reporter Mice: Tools for Visualizing Gene Expression
Looking for a specific transgenic mouse model? Let's discuss your unique research needs in detail. Contact us today to explore how Creative Biolabs can help advance your studies
References
- Lampreht Tratar, Ursa, Simon Horvat, and Maja Cemazar. "Transgenic mouse models in cancer research." Frontiers in oncology 8 (2018): 268. https://doi.org/10.3389/fonc.2018.00268
- Distributed under Open Access license CC BY 4.0 , without modification.
For Research Use Only.
